Sat, Jul 12, 2025
Volume 17, Issue 2 (Summer & Autumn 2020)
ASJ 2020, 17(2): 55-62 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Azari Z, Zamini A, Dabirian S, Mehrdad M, Olfati J, Dadgaran I et al . Cytotoxicity Effect of Hull-Less Seed Pumpkin Extract on Human Papillary Thyroid Cancer Cell Line. ASJ 2020; 17 (2) :55-62
URL: http://anatomyjournal.ir/article-1-222-en.html
URL: http://anatomyjournal.ir/article-1-222-en.html
Zoleikha Azari1 

 , Arash Zamini1
, Arash Zamini1 
 , Sara Dabirian2
, Sara Dabirian2 
 , Mojtaba Mehrdad3
, Mojtaba Mehrdad3 
 , Jamalali Olfati4
, Jamalali Olfati4 
 , Ideh Dadgaran5
, Ideh Dadgaran5 
 , Mohammad Hadi Bahadori1
, Mohammad Hadi Bahadori1 




 , Arash Zamini1
, Arash Zamini1 
 , Sara Dabirian2
, Sara Dabirian2 
 , Mojtaba Mehrdad3
, Mojtaba Mehrdad3 
 , Jamalali Olfati4
, Jamalali Olfati4 
 , Ideh Dadgaran5
, Ideh Dadgaran5 
 , Mohammad Hadi Bahadori1
, Mohammad Hadi Bahadori1 


1- Cellular and Molecular Research Center, Faculty of Medicine, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Pharmaceutical Biotechnology, School of Pharmacy, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Endocrinology, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Horticultural, Faculty of Agriculture Science, Guilan University of Medical Sciences, Rasht,Iran.
5- Medical Educational Research Centre, Guilan University of Medical Sciences, Rasht, Iran.
2- Department of Pharmaceutical Biotechnology, School of Pharmacy, Guilan University of Medical Sciences, Rasht, Iran.
3- Department of Endocrinology, Guilan University of Medical Sciences, Rasht, Iran.
4- Department of Horticultural, Faculty of Agriculture Science, Guilan University of Medical Sciences, Rasht,Iran.
5- Medical Educational Research Centre, Guilan University of Medical Sciences, Rasht, Iran.
Full-Text [PDF 1110 kb]
(1385 Downloads)
| Abstract (HTML) (3627 Views)
Full-Text: (1309 Views)
1. Introduction
Thyroid cancer is the most prevalent endocrine cancer, and the most common type of thyroid cancer is Papillary. Papillary Thyroid Cancer (PTC) comprises about 80% of such malignancies, and its incidence is rising in different populations [1, 2]. In Iran, thyroid cancer accounts for 1.8% of all cancers and 76.1% of endocrine cancers. Furthermore, PTC is the most frequent thyroid cancer type, with an incidence rate of 80%. Epidemiology studies in Iran also documented that the incidence of thyroid cancer in both genders is on the rise [3].
Current chemotherapy drugs, such as doxorubicin, daunorubicin, Paclitaxel (PTX), docetaxel, and cyclophosphamide are used as the first-line treatment for many cancers; however, these drugs have adverse effects, like alopecia and Chemotherapy-Induced Neuropathic Pain (CINP) [4-6]. Paclitaxel, one of the most important members of the taxon family, is a well-known anticancer drug used for treating various cancers [7]. It exerts its cytotoxic effect by arresting mitosis through stabilizing microtubules by preventing their depolymerization [8]. The available chemotherapy drugs are unbeneficial to all cases and have serious adverse effects on human health; thus, finding new anticancer agents with fewer side effects seems imperative. Studies indicated that herbal medicines with anticancer potential are considered as promising therapeutic agents.
Moreover, different plants have anticancer properties [9, 10]. Cucurbita pepo L. (Cucurbitaceae), commonly known as the “pumpkin” seed, has extensive therapeutic properties, including antibacterial, antiviral, anti-inflammatory, analgesic, anti-mutagenic, and anticancer effects. Furthermore, pumpkin seed improves Benign Prostate Hyperplasia (BPH) associated symptoms [11, 12].
Seeds and seed oil of pumpkin are rich in proteins, fithostrol, fatty and non-saturated fatty acids, including linoleic, linolenic, palmitic, stearic, vitamins (A and E), phenol antioxidant compounds, such as carotenoids, lutein, tocopherol, Gama, chlorophyll, as well as elements, like zinc and selenium [11, 13]. In addition, a diet containing high amounts of pumpkin seeds reduced the risk of gastric, breast, lung, and colon cancer [12]. Moreover, different carotenoid pigments in pumpkin seed oil prevent prostate cancer [12]. In a research study, pumpkin extract reduced the weight of s-180 tumors in mice [14]. Also, several base proteins, called MAP2 (MW 2249 Da) and MAP4 (MW 4650 Da) were extracted from pumpkin seed, which has inhibitory effects on the blood cancer of K_562 cells. In addition, there exist other proteins in pumpkin seeds, which limit the proliferation of melanoma [12, 15]. In this study, the anticancer effects of hydro-alcoholic extract of HLSP were examined and compared with those of paclitaxel against human PTC cells in the lab.
2. Materials and Methods
Hull-less seed pumpkin (Cucurbita pepo subsp. pepo var. Styriaka) and human PTC cell line were obtained from VBG Company (Rasht, Iran) and Pasteur Institute of Iran, respectively. All procedures were approved by the Ethics Committee of Guilan University of Medical Sciences.
The PTC cell line was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich), supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich), 100U/mL penicillin (Sigma-Aldrich), and 100μg/mL streptomycin (Sigma-Aldrich). Cell cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. The medium was changed twice a week [16]. The frequency(%) of dead cells was determined by Trypan blue staining [16].
For the preparation of hydro-alcoholic extract, the method described by Navneet Kumar Yadav et al. was followed [17]. Briefly, a measured quantity of 50g of powdered and dried seeds of pumpkin was chopped and soaked in 500mL of ethanol (80%v/v) for 72 h. The suspension was then passed through filter paper and concentrated using a rotary vacuum evaporator at 40°C. Specific concentrations of hydro-alcoholic extracts (1, 20, 50, 100, 200, 800, 1600, 2400, 2800, 6400, and 100000 μg/mL) were prepared with phosphate buffer (pH=7.4). The hydro-alcoholic extracts were sterilized by 0.22 μm microbiological filters and maintained at 4°C before use.
To evaluate the effects of the drug on cells’ viability, Trypan blue staining was used. To conduct Trypan blue staining, after performing the third passage and cell counting by Neubauer slide, the equal number of 80000 cells were added to any six-cell well plate. Then, by adding complete media, the volume of all wells was increased to 2 mL. After 24 h, all wells were first observed and inspected by an inverted microscope for proper growth and the unpollutedness of cells. Next, the prepared concentrations from the hydro-alcoholic extract of HLSP and paclitaxel were separately added to each well. To observe and investigate cell growth inhibition, Trypan blue staining was performed after 24, 48, and 72 h. For the preparation of this stain, 4.0% of Trypan blue powder (Sigma-Aldrich) was added to the sodium phosphate buffer (pH=7.4). To stain wells, each well was initially passaged separately and centrifuged, respectively. Then, 20 µL of the cell suspension was mixed with 20 µL of Trypan blue staining solution, and 10 µL of the resulting mixture was put on Neubauer slide. In this staining, the color of cells with defect membranes was observed as blue, and live cells were observed as completely apparent and colorless. Experiments were performed three times in triplicate.
To detect apoptotic cells, PTC cells were seeded in 96-well plates at a density of 5×10³ cells per well and incubated for 24 h; then, treated by different concentrations of paclitaxel (0.00001-6000 μg/mL) or extract (1-100000μg/mL) for 24, 48, and 72 h. To show the cell death, suspensions from plate cells were prepared and stained after 24, 48, and 72 h. As described by Aline Monezi Montel et al. [18], 50 μL of Acridine Orange/Ethidium Bromide (AO/EB) dye (a mixture of AO (100 μg/mL) (Sigma) and EB (100 μg/ml) in PBS) (Sigma-Aldrich)) was prepared. Cells were observed and counted under an inverted fluorescence microscope (Olympus, IX71, Japan). This experiment was preformed three times in triplicate. AO is absorbed both by living and dead cells. AO enters the cell nucleus and offers a green view of living cell chromatin; however, EB only penetrates the dead cells and gives an orange view to the chromatin of dead cells under the fluorescence microscope.
To distinguish the cell viability after treatment by paclitaxel and the extract, clonogenic assay was performed as described by Jinichi Mori et al. [19]. Briefly, 2 mL of DMEM, containing 2000 cells, was added to six-well plates. They were incubated at 37°C for 24 h. After incubation time, the cells were exposed to different concentrations of paclitaxel (0.00001-20 μg/mL) or the extract (200-6400 μg/mL) for 7 days. After 7 days, the cells were stained by Giemsa dye, as described above. The cell colony images were prepared, and more than 50 cell colonies were counted using a light microscope.
The obtained data were analyzed by SPSS. Statistical comparisons between the study groups were made with one-way Analysis of Variance (ANOVA) and Tukey test. Mean differences were considered significant at P<0.05.
3. Results
According to the results obtained from Trypan blue staining, in terms of the concentration of 1mg/mL of hydro-alcoholic extract, there were no significant differences in the controls between the three examination times (24, 48, and 72 h). However, in the concentration of 200µg/mL, the number of dead cells increased, and the viability of cells declined up to 15%. In the concentration of 800 µg/mL, the viability of cells declined up to 30%, as well. In these concentrations, time had no significant impact on the increase or decrease in cell death. In the concentration of 1600 µg/mL, cell viability was extensively reduced. Moreover, in the concentration of 6400 µg/mL, the viability of cells reached the minimum, and the number of dead cells increased significantly (P≤0.05). In the paclitaxel treated groups, in the concentration of 0.00001 µg/mL, except for the case of 72 h, cell death was not observed; however, with an increase in concentration, a significant reduction occurred in cell viability (P≤0.05) (Figure 1).
.PNG)
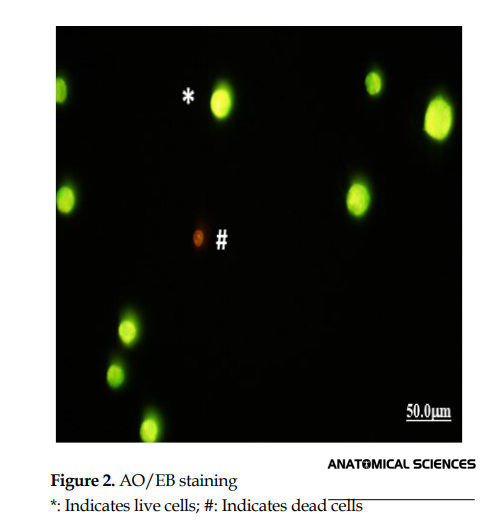
To illustrate the frequency (%) of apoptotic cells, after treating PTC cells by different concentrations of paclitaxel and the HLSP extract, AO/EB staining was carried out. In the AO/EB staining, live cells are specified with green color, and dead cells are specified with orange to red color (Figure 2).
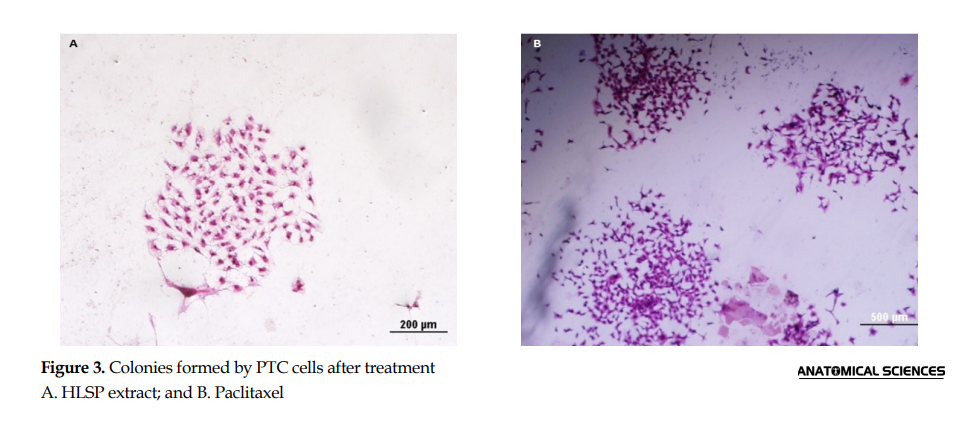
To determine the amount of cell growth and colony forming PTC cells after treatment with the extract, clonogenic assay was used.
Similar to the results of Trypan blue staining and AO/EB staining, results of clonogenic assay showed when the concentration of Paclitaxel or pumpkin hydro-alcoholic extract increased, a significant decrease in the number of colonies was observed in comparison with the control group. The percentage of colonies formed after a week was 47.91% in the concentration of 1600 in the extract compared to 58.75% in the concentration of 0.01 in Paclitaxel (compared to the control group) (P<0.001) (Figure 3 and Figure 4).

4. Discussion
The present study investigated the pharmacological effects of HLSP on the PTC cell line. Due to the presence of some crucial components, such as sterols, polysaccharides, fixed oils, para-aminobenzoic acid, proteins, and peptides, pumpkin has been considered as a valuable health nutrient. Pumpkin seeds are a useful source of protein and essential amino acids, fatty acid, linoleic acid, and some beneficial ions, such as K, Cr, Na, Mg, Zn, Cu, Mo, and Se [20, 21]. Various extracts and fractions obtained from pumpkin have robust antioxidant activity and profoundly affect the prevention and treatment of some endocrine and vascular diseases [22, 23]. Pumpkin seed and its associated oil contain a rich source of tocopherol (vitamin E) (as an essential antioxidant) [24]. In addition, administrated pumpkin extract increased the serous and hepatic activity of Superoxide Dismutase (SOD) and decreased the lipid peroxidation of cell membranes [12]. Furthermore, pumpkin extract could increase the SOD and decrease the malonaldehyde content in tumor-containing mice serum [25].
In the thyroid gland, epithelial cells create some reactive oxygen species, i.e. required for the synthesis of T3 and T4 hormones. Nevertheless, when the aforementioned cells produce excessive ROS, they cause toxic effects on thyroid cells [26, 27]. Among environmental factors causing or predisposing to thyroid cancer, oxidative stress plays an important role. Oxidative stress is a biochemical condition characterized by the accumulation of ROSs, such as superoxide anion radical, hydroxyl radical, hydrogen peroxide, and imbalance between pro-oxidant and antioxidant compounds [28]. Wang et al. investigated the total oxidant/antioxidant status in the sera of patients with thyroid cancers. They suggested that oxidant parameters increased, and antioxidant parameters decreased in thyroid cancer [29]. Muzza et al. surveyed the oxidative stress and the subcellular localization of the telomerase reverse transcriptase in PTC. Their data demonstrated that the intracellular H2O2 (as oxidant parameters) is significantly higher in PTCs than in healthy thyroid tissues [30]. Furthermore, they concluded that mitochondrial oxidative stress was not markedly different in normal and tumor tissues [30]. Given the crucial role of oxidative stress in the pathogenesis of PTC and the proven antioxidant activity of pumpkin seeds, selecting pumpkin seed in the present study was precious.
Programmed cell death, or apoptosis, is a mechanism by which cells undergo death to regulate cell propagation or in reply to genomic damage. This study surveyed the anticancer activity of HLSP on the PTC cell line employing apoptosis. Understanding apoptosis has provided a critical basis for cancer therapies that can induce tumor cell death or sensitize them to chemotherapy and radiation therapy [31-33]. In the aforementioned novel method of cancer therapy, some agents target the extrinsic pathway of apoptosis, like tumor necrosis factor-related to apoptosis, and some agents target the intrinsic Bcl-2 family pathway [31].
In the present study, AO/EB staining was used to depict nuclear changes and apoptotic body formation as a typical characteristic of apoptosis. To avoid the misidentification of live and dead cells, special attention was paid to some key features of these cells. Both live and dead cells were stained with AO dye; however, EB stained were the only cells that lost cell membrane integration. In this staining, live cells would appear in green. Early apoptotic cells are apparent green accompanied by nuclear bright dots as a consequence of genomic condensation and nuclear fragmentation process. Late apoptotic cells are apparent orange (due to interaction with EB dye) [34]. AO/EB staining results suggested that the hydro-alcoholic extract of HLSP in the concentration of ≥100μl/mL induced significant apoptosis in the PTC cultured cell (P<0.05). In addition to the aforementioned staining, we performed clonogenic assay to ensure the existence of apoptosis. The data obtained from clonogenic assay are consistent with those of AO/EB staining. Additionally, based on our perceptual analysis, the number of apoptotic cells in the treatment group with concentrations of 200, 800, 2400, and 2800 μl/mL were more than that of the controls.
According to the literature, the present study was the first to investigate the effects of HLSP on thyroid cancer. Previously, pumpkin therapeutic effects on breast, blood, and prostate cancer have been proven. Richter et al. investigated the phytoestrogen extracts isolated from pumpkin seeds on breast cancer. Their results highlighted a potential role of pumpkin seeds in breast cancer prevention and treatment [35]. As previously mentioned, the inhibitory action of pumpkin is also proven on leukemia and prostate cancer.
5. Conclusion
Hydro-alcoholic extract of HLSP induced PTC cell apoptosis in vitro. The results, as mentioned above, could highlight the potential role of HLSP in PTC prevention and treatment. However, for making precise decisions about the effect of HLSP on PTC, more molecular investigations are required.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Guilan University of Medical Sciences (Code: ???).
Funding
This research was supported by the research project (Grant No.: 3/132/8162/P) of the Guilan University of Medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
There are no conflicts of interest to be declared.
Acknowledgments
Guilan University of Medical Sciences provided (Grant no.: 3/132/8162/P). We would like to thank the Cellular and Molecular Research Center at the School of Medicine.
Refrences:
Thyroid cancer is the most prevalent endocrine cancer, and the most common type of thyroid cancer is Papillary. Papillary Thyroid Cancer (PTC) comprises about 80% of such malignancies, and its incidence is rising in different populations [1, 2]. In Iran, thyroid cancer accounts for 1.8% of all cancers and 76.1% of endocrine cancers. Furthermore, PTC is the most frequent thyroid cancer type, with an incidence rate of 80%. Epidemiology studies in Iran also documented that the incidence of thyroid cancer in both genders is on the rise [3].
Current chemotherapy drugs, such as doxorubicin, daunorubicin, Paclitaxel (PTX), docetaxel, and cyclophosphamide are used as the first-line treatment for many cancers; however, these drugs have adverse effects, like alopecia and Chemotherapy-Induced Neuropathic Pain (CINP) [4-6]. Paclitaxel, one of the most important members of the taxon family, is a well-known anticancer drug used for treating various cancers [7]. It exerts its cytotoxic effect by arresting mitosis through stabilizing microtubules by preventing their depolymerization [8]. The available chemotherapy drugs are unbeneficial to all cases and have serious adverse effects on human health; thus, finding new anticancer agents with fewer side effects seems imperative. Studies indicated that herbal medicines with anticancer potential are considered as promising therapeutic agents.
Moreover, different plants have anticancer properties [9, 10]. Cucurbita pepo L. (Cucurbitaceae), commonly known as the “pumpkin” seed, has extensive therapeutic properties, including antibacterial, antiviral, anti-inflammatory, analgesic, anti-mutagenic, and anticancer effects. Furthermore, pumpkin seed improves Benign Prostate Hyperplasia (BPH) associated symptoms [11, 12].
Seeds and seed oil of pumpkin are rich in proteins, fithostrol, fatty and non-saturated fatty acids, including linoleic, linolenic, palmitic, stearic, vitamins (A and E), phenol antioxidant compounds, such as carotenoids, lutein, tocopherol, Gama, chlorophyll, as well as elements, like zinc and selenium [11, 13]. In addition, a diet containing high amounts of pumpkin seeds reduced the risk of gastric, breast, lung, and colon cancer [12]. Moreover, different carotenoid pigments in pumpkin seed oil prevent prostate cancer [12]. In a research study, pumpkin extract reduced the weight of s-180 tumors in mice [14]. Also, several base proteins, called MAP2 (MW 2249 Da) and MAP4 (MW 4650 Da) were extracted from pumpkin seed, which has inhibitory effects on the blood cancer of K_562 cells. In addition, there exist other proteins in pumpkin seeds, which limit the proliferation of melanoma [12, 15]. In this study, the anticancer effects of hydro-alcoholic extract of HLSP were examined and compared with those of paclitaxel against human PTC cells in the lab.
2. Materials and Methods
Hull-less seed pumpkin (Cucurbita pepo subsp. pepo var. Styriaka) and human PTC cell line were obtained from VBG Company (Rasht, Iran) and Pasteur Institute of Iran, respectively. All procedures were approved by the Ethics Committee of Guilan University of Medical Sciences.
The PTC cell line was cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich), supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich), 100U/mL penicillin (Sigma-Aldrich), and 100μg/mL streptomycin (Sigma-Aldrich). Cell cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2. The medium was changed twice a week [16]. The frequency(%) of dead cells was determined by Trypan blue staining [16].
For the preparation of hydro-alcoholic extract, the method described by Navneet Kumar Yadav et al. was followed [17]. Briefly, a measured quantity of 50g of powdered and dried seeds of pumpkin was chopped and soaked in 500mL of ethanol (80%v/v) for 72 h. The suspension was then passed through filter paper and concentrated using a rotary vacuum evaporator at 40°C. Specific concentrations of hydro-alcoholic extracts (1, 20, 50, 100, 200, 800, 1600, 2400, 2800, 6400, and 100000 μg/mL) were prepared with phosphate buffer (pH=7.4). The hydro-alcoholic extracts were sterilized by 0.22 μm microbiological filters and maintained at 4°C before use.
To evaluate the effects of the drug on cells’ viability, Trypan blue staining was used. To conduct Trypan blue staining, after performing the third passage and cell counting by Neubauer slide, the equal number of 80000 cells were added to any six-cell well plate. Then, by adding complete media, the volume of all wells was increased to 2 mL. After 24 h, all wells were first observed and inspected by an inverted microscope for proper growth and the unpollutedness of cells. Next, the prepared concentrations from the hydro-alcoholic extract of HLSP and paclitaxel were separately added to each well. To observe and investigate cell growth inhibition, Trypan blue staining was performed after 24, 48, and 72 h. For the preparation of this stain, 4.0% of Trypan blue powder (Sigma-Aldrich) was added to the sodium phosphate buffer (pH=7.4). To stain wells, each well was initially passaged separately and centrifuged, respectively. Then, 20 µL of the cell suspension was mixed with 20 µL of Trypan blue staining solution, and 10 µL of the resulting mixture was put on Neubauer slide. In this staining, the color of cells with defect membranes was observed as blue, and live cells were observed as completely apparent and colorless. Experiments were performed three times in triplicate.
To detect apoptotic cells, PTC cells were seeded in 96-well plates at a density of 5×10³ cells per well and incubated for 24 h; then, treated by different concentrations of paclitaxel (0.00001-6000 μg/mL) or extract (1-100000μg/mL) for 24, 48, and 72 h. To show the cell death, suspensions from plate cells were prepared and stained after 24, 48, and 72 h. As described by Aline Monezi Montel et al. [18], 50 μL of Acridine Orange/Ethidium Bromide (AO/EB) dye (a mixture of AO (100 μg/mL) (Sigma) and EB (100 μg/ml) in PBS) (Sigma-Aldrich)) was prepared. Cells were observed and counted under an inverted fluorescence microscope (Olympus, IX71, Japan). This experiment was preformed three times in triplicate. AO is absorbed both by living and dead cells. AO enters the cell nucleus and offers a green view of living cell chromatin; however, EB only penetrates the dead cells and gives an orange view to the chromatin of dead cells under the fluorescence microscope.
To distinguish the cell viability after treatment by paclitaxel and the extract, clonogenic assay was performed as described by Jinichi Mori et al. [19]. Briefly, 2 mL of DMEM, containing 2000 cells, was added to six-well plates. They were incubated at 37°C for 24 h. After incubation time, the cells were exposed to different concentrations of paclitaxel (0.00001-20 μg/mL) or the extract (200-6400 μg/mL) for 7 days. After 7 days, the cells were stained by Giemsa dye, as described above. The cell colony images were prepared, and more than 50 cell colonies were counted using a light microscope.
The obtained data were analyzed by SPSS. Statistical comparisons between the study groups were made with one-way Analysis of Variance (ANOVA) and Tukey test. Mean differences were considered significant at P<0.05.
3. Results
According to the results obtained from Trypan blue staining, in terms of the concentration of 1mg/mL of hydro-alcoholic extract, there were no significant differences in the controls between the three examination times (24, 48, and 72 h). However, in the concentration of 200µg/mL, the number of dead cells increased, and the viability of cells declined up to 15%. In the concentration of 800 µg/mL, the viability of cells declined up to 30%, as well. In these concentrations, time had no significant impact on the increase or decrease in cell death. In the concentration of 1600 µg/mL, cell viability was extensively reduced. Moreover, in the concentration of 6400 µg/mL, the viability of cells reached the minimum, and the number of dead cells increased significantly (P≤0.05). In the paclitaxel treated groups, in the concentration of 0.00001 µg/mL, except for the case of 72 h, cell death was not observed; however, with an increase in concentration, a significant reduction occurred in cell viability (P≤0.05) (Figure 1).
.PNG)
To illustrate the frequency (%) of apoptotic cells, after treating PTC cells by different concentrations of paclitaxel and the HLSP extract, AO/EB staining was carried out. In the AO/EB staining, live cells are specified with green color, and dead cells are specified with orange to red color (Figure 2).

To determine the amount of cell growth and colony forming PTC cells after treatment with the extract, clonogenic assay was used.
Similar to the results of Trypan blue staining and AO/EB staining, results of clonogenic assay showed when the concentration of Paclitaxel or pumpkin hydro-alcoholic extract increased, a significant decrease in the number of colonies was observed in comparison with the control group. The percentage of colonies formed after a week was 47.91% in the concentration of 1600 in the extract compared to 58.75% in the concentration of 0.01 in Paclitaxel (compared to the control group) (P<0.001) (Figure 3 and Figure 4).


4. Discussion
The present study investigated the pharmacological effects of HLSP on the PTC cell line. Due to the presence of some crucial components, such as sterols, polysaccharides, fixed oils, para-aminobenzoic acid, proteins, and peptides, pumpkin has been considered as a valuable health nutrient. Pumpkin seeds are a useful source of protein and essential amino acids, fatty acid, linoleic acid, and some beneficial ions, such as K, Cr, Na, Mg, Zn, Cu, Mo, and Se [20, 21]. Various extracts and fractions obtained from pumpkin have robust antioxidant activity and profoundly affect the prevention and treatment of some endocrine and vascular diseases [22, 23]. Pumpkin seed and its associated oil contain a rich source of tocopherol (vitamin E) (as an essential antioxidant) [24]. In addition, administrated pumpkin extract increased the serous and hepatic activity of Superoxide Dismutase (SOD) and decreased the lipid peroxidation of cell membranes [12]. Furthermore, pumpkin extract could increase the SOD and decrease the malonaldehyde content in tumor-containing mice serum [25].
In the thyroid gland, epithelial cells create some reactive oxygen species, i.e. required for the synthesis of T3 and T4 hormones. Nevertheless, when the aforementioned cells produce excessive ROS, they cause toxic effects on thyroid cells [26, 27]. Among environmental factors causing or predisposing to thyroid cancer, oxidative stress plays an important role. Oxidative stress is a biochemical condition characterized by the accumulation of ROSs, such as superoxide anion radical, hydroxyl radical, hydrogen peroxide, and imbalance between pro-oxidant and antioxidant compounds [28]. Wang et al. investigated the total oxidant/antioxidant status in the sera of patients with thyroid cancers. They suggested that oxidant parameters increased, and antioxidant parameters decreased in thyroid cancer [29]. Muzza et al. surveyed the oxidative stress and the subcellular localization of the telomerase reverse transcriptase in PTC. Their data demonstrated that the intracellular H2O2 (as oxidant parameters) is significantly higher in PTCs than in healthy thyroid tissues [30]. Furthermore, they concluded that mitochondrial oxidative stress was not markedly different in normal and tumor tissues [30]. Given the crucial role of oxidative stress in the pathogenesis of PTC and the proven antioxidant activity of pumpkin seeds, selecting pumpkin seed in the present study was precious.
Programmed cell death, or apoptosis, is a mechanism by which cells undergo death to regulate cell propagation or in reply to genomic damage. This study surveyed the anticancer activity of HLSP on the PTC cell line employing apoptosis. Understanding apoptosis has provided a critical basis for cancer therapies that can induce tumor cell death or sensitize them to chemotherapy and radiation therapy [31-33]. In the aforementioned novel method of cancer therapy, some agents target the extrinsic pathway of apoptosis, like tumor necrosis factor-related to apoptosis, and some agents target the intrinsic Bcl-2 family pathway [31].
In the present study, AO/EB staining was used to depict nuclear changes and apoptotic body formation as a typical characteristic of apoptosis. To avoid the misidentification of live and dead cells, special attention was paid to some key features of these cells. Both live and dead cells were stained with AO dye; however, EB stained were the only cells that lost cell membrane integration. In this staining, live cells would appear in green. Early apoptotic cells are apparent green accompanied by nuclear bright dots as a consequence of genomic condensation and nuclear fragmentation process. Late apoptotic cells are apparent orange (due to interaction with EB dye) [34]. AO/EB staining results suggested that the hydro-alcoholic extract of HLSP in the concentration of ≥100μl/mL induced significant apoptosis in the PTC cultured cell (P<0.05). In addition to the aforementioned staining, we performed clonogenic assay to ensure the existence of apoptosis. The data obtained from clonogenic assay are consistent with those of AO/EB staining. Additionally, based on our perceptual analysis, the number of apoptotic cells in the treatment group with concentrations of 200, 800, 2400, and 2800 μl/mL were more than that of the controls.
According to the literature, the present study was the first to investigate the effects of HLSP on thyroid cancer. Previously, pumpkin therapeutic effects on breast, blood, and prostate cancer have been proven. Richter et al. investigated the phytoestrogen extracts isolated from pumpkin seeds on breast cancer. Their results highlighted a potential role of pumpkin seeds in breast cancer prevention and treatment [35]. As previously mentioned, the inhibitory action of pumpkin is also proven on leukemia and prostate cancer.
5. Conclusion
Hydro-alcoholic extract of HLSP induced PTC cell apoptosis in vitro. The results, as mentioned above, could highlight the potential role of HLSP in PTC prevention and treatment. However, for making precise decisions about the effect of HLSP on PTC, more molecular investigations are required.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Guilan University of Medical Sciences (Code: ???).
Funding
This research was supported by the research project (Grant No.: 3/132/8162/P) of the Guilan University of Medical Sciences.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
There are no conflicts of interest to be declared.
Acknowledgments
Guilan University of Medical Sciences provided (Grant no.: 3/132/8162/P). We would like to thank the Cellular and Molecular Research Center at the School of Medicine.
Refrences:
- Zhong J, Lei J, Jiang K, Li Z, Gong R, Zhu J. Synchronous papillary thyroid carcinoma and breast ductal carcinoma: A rare case report and literature review. Medicine. 2017; 96(7):e6114. [DOI:10.1097/MD.0000000000006114] [PMID] [PMCID]
- Yu W, Zhu L, Xu G, Song Y, Li G, Zhang N. Potential role of carbon nanoparticles in protection of parathyroid glands in patients with papillary thyroid cancer. Medicine. 2016; 95(42):e5002. [DOI:10.1097/MD.0000000000005002] [PMID] [PMCID]
- Hajizadeh N, Pourhoseingholi MA, Baghestani AR. Incidence rate of thyroid cancer in Iranian population, trend analysis from 2003 to 2009. International Journal of Epidemiologic Research. 2015; 2(1):12-7.
- Qu CP, Sun GX, Yang SQ, Tian J, Si JG, Wang YF. Toxicities of different first-line chemotherapy regimens in the treatment of advanced ovarian cancer: A network meta-analysis. Medicine. 2017; 96(2):e5797. [DOI:10.1097/MD.0000000000005797] [PMID] [PMCID]
- Masocha W, Parvathy SS. Preventative and therapeutic effects of a GABA transporter 1 inhibitor administered systemically in a mouse model of paclitaxel-induced neuropathic pain. PeerJ. 2016; 4:e2798. [DOI:10.7717/peerj.2798] [PMID] [PMCID]
- Fehr M, Welter J, Sell W, Jung R, Felberbaum R. Sensor-controlled scalp cooling to prevent chemotherapy-induced alopecia in female cancer patients. Current Oncology. 2016; 23(6):e576. [DOI:10.3747/co.23.3200] [PMID] [PMCID]
- Wang N, Wang Z, Nie S, Song L, He T, Yang S, et al. Biodegradable polymeric micelles coencapsulating paclitaxel and honokiol: A strategy for breast cancer therapy in vitro and in vivo. International Journal Of Nanomedicine. 2017; 12:1499-514. [DOI:10.2147/IJN.S124843] [PMID] [PMCID]
- Wang X, Beitler JJ, Wang H, Lee MJ, Huang W, Koenig L, et al., Honokiol enhances paclitaxel efficacy in multi-drug resistant human cancer model through the induction of apoptosis. PLOS One. 2014; 9(2):e86369. [DOI:10.1371/journal.pone.0086369] [PMID] [PMCID]
- Bhandari J, Muhammad BT, Thapa P, Shrestha BG. Study of phytochemical, anti-microbial, anti-oxidant, and anti-cancer properties of Allium wallichii. BMC Complementary and Alternative Medicine. 2017; 17(1):102. [DOI:10.1186/s12906-017-1622-6] [PMID] [PMCID]
- Tapadiya G, Kale MA, Saboo S. Phytochemical characterization, anti-cancer and antimicrobial activity of isolated fractions of Alysicarpus vaginalis. Bangladesh Journal of Pharmacology. 2017; 12(1):77-83. [DOI:10.3329/bjp.v12i1.29995]
- Peiretti PG, Meineri G, Gai F, Longato E, Amarowicz R. Antioxidative activities and phenolic compounds of pumpkin (Cucurbita pepo) seeds and amaranth (Amaranthus caudatus) grain extracts. Natural Product Research. 2017; 31(18):2178-82. [DOI:10.1080/14786419.2017.1278597] [PMID]
- Yadav M, Jain S, Tomar R, Prasad GBKS, Yadav H. Medicinal and biological potential of pumpkin: An updated review. Nutrition Research Reviews. 2010; 23(2):184-90. [DOI:10.1017/S0954422410000107] [PMID]
- Zhao XJ, Chen YL, Zhang W, Liu Z, Zhou H. Intervention of pumpkin seed oil on metabolic disease revealed by metabonomics and transcript profile. Journal of the Science of Food and Agriculture. 2017; 97(4):1158-63. [DOI:10.1002/jsfa.7842] [PMID]
- Pan HZ, Qiu XH, Li H, Jin J, Yu C, Zhao J. Effect of pumpkin extracts on tumor growth inhibition in S180-bearing mice. Practical Preventive Medicine. 2005; 12:745-7.
- Xia HC, Li F, Li Z, Zhang ZC. Purification and characterization of Moschatin, a novel type I ribosome-inactivating protein from the mature seeds of pumpkin (Cucurbita moschata), and preparation of its immunotoxin against human melanoma cells. Cell Research. 2003; 13(5):369-74. [DOI:10.1038/sj.cr.7290182] [PMID]
- Catunda RQ, Vieira JRC, Oliveira EB, da Silva EC, Brasil VLM, Perez DEC. Citotoxicity evaluation of three dental adhesives on vero cells in vitro. Journal of Clinical and Experimental Dentistry. 2017; 9(1):e61-6. [DOI:10.4317/jced.53039] [PMID] [PMCID]
- Yadav NK, Arya RK, Dev K, Sharma C, Hossain Z, Meena S, et al. Alcoholic extract of Eclipta alba shows in vitro antioxidant and anticancer activity without exhibiting toxicological effects. Oxidative Medicine and Cellular Longevity. 2017; 2017:9094641. [DOI:10.1155/2017/9094641] [PMID] [PMCID]
- Montel AM, Santos RGD, Costa PRD, Silveira-Lacerda EDP, Batista AA, Santos WGD. Neutron activation increases activity of ruthenium-based complexes and induces cell death in glioma cells independent of p53 tumor suppressor gene. BioMetals. 2017; 30(2):295-305. [DOI:10.1007/s10534-017-0006-1] [PMID] [PMCID]
- Mori J, Tanikawa C, Ohnishi N, Funauchi Y, Toyoshima O, Ueda K, et al. EPSIN 3, a novel p53 target, regulates the apoptotic pathway and gastric carcinogenesis. Neoplasia. 2017; 19(3):185-95. [DOI:10.1016/j.neo.2016.12.010] [PMID] [PMCID]
- Glew RH, Glew RS, ChuangLT, Huang YS, Millson M, Constans D, et al. Amino acid, mineral and fatty acid content of pumpkin seeds (Cucurbita spp) and Cyperus esculentus nuts in the Republic of Niger. Plant Foods for Human Nutrition. 2006; 61(2):49-54. [DOI:10.1007/s11130-006-0010-z] [PMID]
- Nwokolo E, Sim JS. Nutritional assessment of defatted oil meals of melon (Colocynthis citrullus L.) and fluted pumpkin (Telfaria occidentalis Hook) by chick assay. Journal of the Science of Food and Agriculture. 1987; 38(3):237-46. [DOI:10.1002/jsfa.2740380307]
- Xia T, Wang Q. Antihyperglycemic effect of Cucurbita ficifolia fruit extract in streptozotocin-induced diabetic rats. Fitoterapia. 2006; 77(7-8):530-3. [DOI:10.1016/j.fitote.2006.06.008] [PMID]
- Abdel Aal FS. The Protective Effect of Pumpkin Seed Oil on Azathioprine-Induced Hepatic Toxicity in Adult Male Albino Rats: Histological and Immunohistochemical Study. Basic Sciences of Medicine. 2014; 3(4):85-100. [DOI:10.5923/j.medicine.20140304.03]
- Tokudome Y, Imaeda N, Ikeda M, Kitagawa I, Fujiwara N, Tokudome S. Foods contributing to absolute intake and variance in intake of fat, fatty acids and cholesterol in middle-aged Japanese. Journal of Epidemiology. 1999; 9(2):78-90. [DOI:10.2188/jea.9.78] [PMID]
- Caili F, Huan S, Quanhong L. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods for Human Nutrition. 2006; 61(2):70-7.
- Praveen KJ, Deepa M, Julius A, Nadiger HA. Study on thyroid status and oxidants in smokers and alcoholics. Journal of Evolution of Medical and Dental Sciences. 2013; 2(36):6982-8. [DOI:10.14260/jemds/1243]
- Asci A, Bulus D, Andiran N, Kocer-Gumusel B, editors. Evaluation of the relation between Thyroid Dysfunction and Oxidant/Antioxidant status in obese children. European Society for Paediatric Endocrinology. 2014; 82 P-D-3-3-659
- Sies H, Berndt C, Jones DP. Oxidative stress. Annual Review of Biochemistry. 2017; 86:715-48. [DOI:10.1146/annurev-biochem-061516-045037] [PMID]
- Wang D, Feng J, Zeng P, Yang Y, Luo J, Yang Y. Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocrine-related cancer. 2011; 18(6):773-82. [DOI:10.1530/ERC-11-0230] [PMID] [PMCID]
- Muzza M, Colombo C, Cirello V, Perrino M, Vicentini L, Fugazzola L. Oxidative stress and the subcellular localization of the telomerase reverse transcriptase (TERT) in papillary thyroid cancer. Molecular and Cellular Endocrinology. 2016; 431:54-61. [DOI:10.1016/j.mce.2016.05.005] [PMID]
- Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA: A cancer journal for clinicians. 2005; 55(3):178-94. [DOI:10.3322/canjclin.55.3.178] [PMID]
- Li-Weber M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer letters. 2013; 332(2):304-12. [DOI:10.1016/j.canlet.2010.07.015] [PMID]
- Rathore R, McCallum JE, Varghese E, Florea AM, Büsselberg D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis. 2017; 22(7):898-919. [DOI:10.1007/s10495-017-1375-1] [PMID] [PMCID]
- Kasibhatla S, Amarante-Mendes GP, Finucane D, Brunner T, Bossy-Wetzel E, Green DR. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harbor Protocols. 2006; 2006(3):pdb-rot4493. [DOI:10.1101/pdb.prot4493] [PMID]
- Richter D, Abaruza S, Chrobak M, Vrekoussis T, Weissenbacher T, Kuhn C, et al., Effects of phytoestrogen extracts isolated from pumpkin seeds on estradiol production and ER/PR expression in breast cancer and trophoblast tumor cells. Nutrition and Cancer. 2013; 65(5):739-45. [DOI:10.1080/01635581.2013.797000] [PMID]
Type of Study: Original |
Subject:
Morphometry
Received: 2018/06/30 | Accepted: 2018/07/5 | Published: 2019/04/20
Received: 2018/06/30 | Accepted: 2018/07/5 | Published: 2019/04/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |

