Thu, Dec 4, 2025
Volume 20, Issue 2 (Summer & Autumn 2023)
ASJ 2023, 20(2): 45-54 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Khaledi S, Najafpour Pitka M, Hoseini Z S, Ghorbanlou M, Shirazi R. Study the Effects of N-acetyl Cysteine and Melatonin on the Quality of Oocyte and Embryo in Women With Polycystic Ovary Syndrome. ASJ 2023; 20 (2) :45-54
URL: http://anatomyjournal.ir/article-1-224-en.html
URL: http://anatomyjournal.ir/article-1-224-en.html
Sajed Khaledi1 

 , Maryam Najafpour Pitka2
, Maryam Najafpour Pitka2 

 , Zahra Sadat Hoseini3
, Zahra Sadat Hoseini3 

 , Mehrdad Ghorbanlou4
, Mehrdad Ghorbanlou4 

 , Reza Shirazi5
, Reza Shirazi5 




 , Maryam Najafpour Pitka2
, Maryam Najafpour Pitka2 

 , Zahra Sadat Hoseini3
, Zahra Sadat Hoseini3 

 , Mehrdad Ghorbanlou4
, Mehrdad Ghorbanlou4 

 , Reza Shirazi5
, Reza Shirazi5 


1- Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
2- Department of Genetics, Faculty of Life Sciences, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran.
3- Department of Biology, Faculty of Genetics, Arsanjan Branch, Islamic Azad University, Arsanjan, Iran.
4- Department of Anatomical Sciences, School of Medicine, Iran University of Medical Science, Tehran, Iran.
5- Department of Anatomy, School of Medical Sciences, Biomedical & Health, UNSW Sydney, Sydney, Australia.
2- Department of Genetics, Faculty of Life Sciences, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran.
3- Department of Biology, Faculty of Genetics, Arsanjan Branch, Islamic Azad University, Arsanjan, Iran.
4- Department of Anatomical Sciences, School of Medicine, Iran University of Medical Science, Tehran, Iran.
5- Department of Anatomy, School of Medical Sciences, Biomedical & Health, UNSW Sydney, Sydney, Australia.
Full-Text [PDF 659 kb]
(289 Downloads)
| Abstract (HTML) (1890 Views)
Full-Text: (1594 Views)
Introduction
With a 15% incidence in couples, infertility is a complex disorder caused by several factors [1]. One of the main causes of infertility in women is polycystic ovary syndrome (PCOS), affecting about 10-15% of women before menopause [2]. PCOS is also linked to irregular ovulation, insulin resistance, and elevated androgen levels, which can result in metabolic complications, including diabetes and cardiovascular diseases [3]. Oocyte quality is a critical factor in the success of assisted reproductive technologies. During ovulation, women with PCOS, typically exhibit a higher quantity of oocytes. However, these oocytes frequently possess inferior quality, resulting in diminished rates of fertilization, cleavage, and implantation [4]. Chronic inflammation, increased oxidative stress, and the subsequent increased cell apoptosis lead to follicular growth disorders and an increased number of atretic follicles in PCOS patients [5, 6]. An imbalance between prooxidants and antioxidants ultimately increases the apoptosis of granulosa cells in PCOS patients and causes symptoms of the disease [7]. Oxidative stress and apoptosis can be reduced by using antioxidants.
Melatonin (N-acetyl-5-methoxytryptamine) is primarily produced by the pineal gland at night. However, it can also be found in other organs, including retina, extraorbital lacrimal gland, Harderian gland, gastrointestinal tract’s enterochromaffin cells, and gut and bone marrow cells. This hormone plays a crucial role in regulating various central and peripheral functions associated with circadian rhythms and reproductive processes [8, 9]. As an indirect antioxidant, Melatonin influences biological processes indirectly by promoting antioxidative enzymes and suppressing prooxidative enzymes. In addition, it serves as a potent direct scavenger of free radicals [10].
N-acetylcysteine (NAC), as a mucolytic agent derived from the amino acid L-cysteine, is a strong antioxidant, destroying free radicals by being converted into metabolites which stimulate glutathione production. Insulin receptors and insulin secretion are affected by NAC. It also increases stored glucose [11]. NAC is an antioxidant, anti-inflammatory, and anti-apoptotic agent, contributing to maintaining vascular integrity and exhibiting immunological effects [12]. It may represent a promising strategy for enhancing or stimulating ovulation in women experiencing chronic anovulation, such as those with PCOS.
In this study, we aim to examine the impacts of NAC, melatonin, and their combination on the quality of embryos and oocytes, in addition to the biochemical parameters present in the follicular fluid (FF), in women with PCOS candidate for intracytoplasmic sperm injection (ICSI). We hypothesized that the concurrent use of melatonin and NAC can enhance insulin sensitivity and promote ovulation in these women.
Materials and Methods
Study design and participants
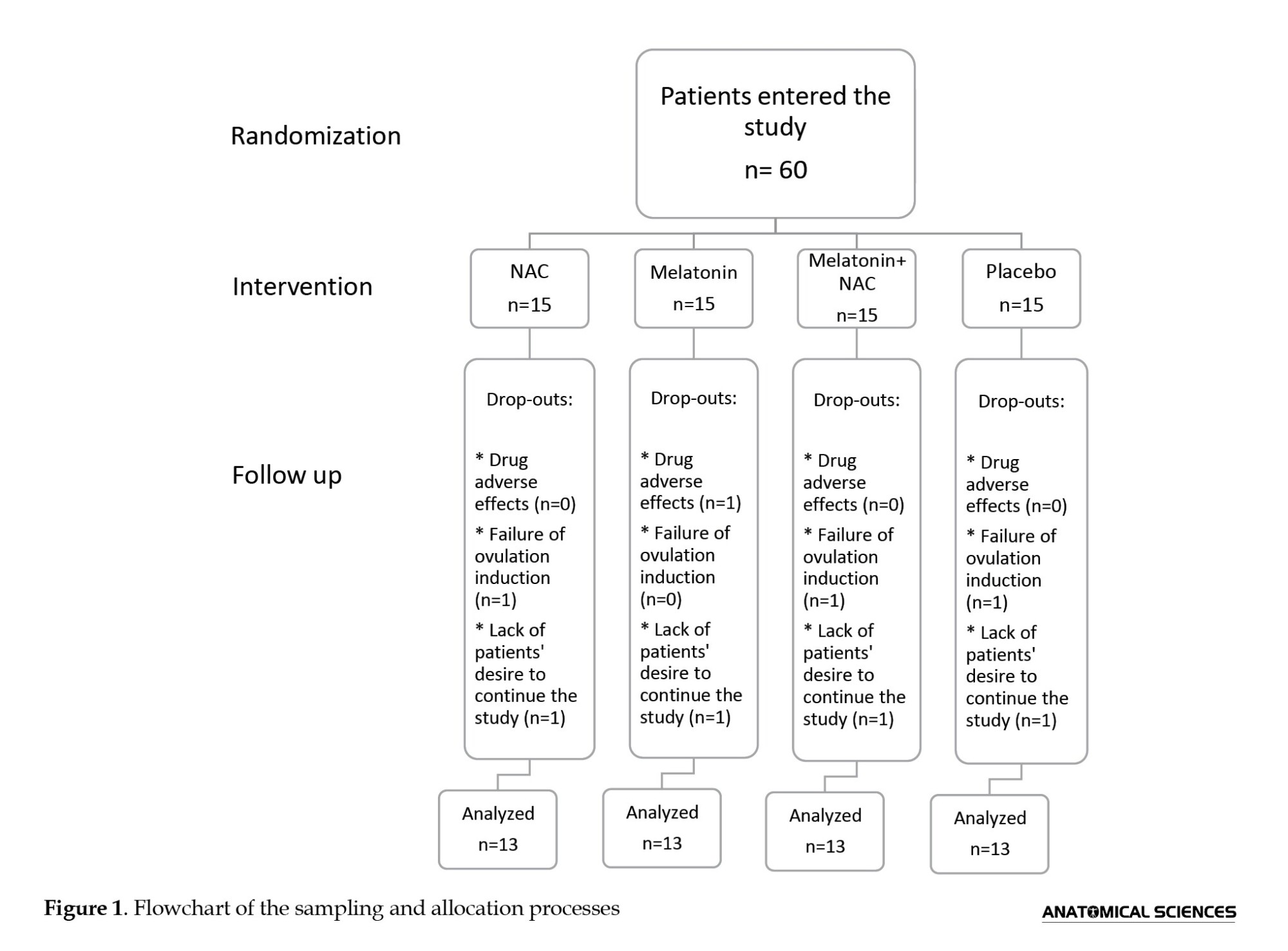
This is a randomized placebo-controlled clinical trial conducted on 60 Iranian women aged 25-35 referred to the infertility clinic of Shahid Akbar Abadi Hospital in Tehran, Iran, from May 2015 to August 2017, who were candidates for ICSI due to PCOS-related infertility. The sample size was determined based on previous studies [13, 14]. Patients were required to meet the diagnostic criteria for PCOS defined by the 2003 Rotterdam Consensus Workshop [15], which is based on the presence of at least two of the following criteria: Chronic oligo- or an-ovulation, clinical or biochemical signs of hyperandrogenism, and polycystic ovaries as observed through ultrasound examination. Exclusion criteria were hypersensitivity to melatonin or NAC, infertility unrelated to anovulation, male infertility, pelvic organic pathologies, congenital adrenal hyperplasia, thyroid dysfunction, Cushing’s syndrome, hyperprolactinaemia, androgen-secreting tumors, diabetes mellitus, the use of medications that affect carbohydrate metabolism and hormonal analogues except for progesterone in two months prior to inclusion, or severe liver or kidney dysfunction. Semen specimens from male partners were evaluated in accordance with World Health Organization (WHO) guidelines [16], and women with partners having irregular semen characteristics were not included in the study. Lack of cooperation, unwillingness to continue the study, and failure of ovulation induction were the reasons for excluding eight women from the study. Finally, 52 patients underwent analysis (Figure 1). All participants were asked not to make any modifications in their regular bodily activity and food plan, and not to use any new medications during the study.
Treatment protocol
The patients were divided into four groups of 15, (i) placebo group, receiving an oral rehydration solution (Razi, Tehran, Iran) three times daily; (ii) melatonin group, receiving melatonin (KAL, Park City, UT, USA) 3 mg per day at 10:00 PM; (ii) NAC group, receiving 600 mg NAC (Batch no. 6N5483; Holzkrichen, Bavaria, Germany) three times daily; and (iv) melatonin+NAC group, receiving the same doses of melatonin and NAC simultaneously. A midwife administered the medication to the patients, ensuring that both the patients and the physicians remained unaware of the treatment protocols. The dosage and duration of NAC treatment were determined based on prior research [14, 17]. All treatments were administered for 40 days, commencing from the fifth day of previous menstrual cycle until the day designated for oocyte aspiration. In addition, all participants were prescribed oral contraceptive pills (OCP) for 21 days, which initiated concurrently with the administration of the placebo, melatonin, NAC, or the melatonin+NAC on the fifth day following menstruation, prior to the start of the treatment cycle. During the study, patients did not show any side effects except one in the melatonin group who had a headache.
Sample collection
The Long protocol [15] was used to stimulate ovulation. Among the analogues of gonadotropin-releasing hormone (GnRH), triptorelin (Decapeptyl; Ferring GmbH, Wittland 11, D-24109 Kiel, Germany) or an antagonist of the GnRH, cetrorelix (Cetrotide; Kantstrasse 2, D-33790 Halle, Germany) was used according to the physician’s opinion. Triptorelin 0.14 mg for 14 days and cetrorelix 0.25 mg/day were injected to observe at least one follicle or more with a size of 14 mm by transvaginal ultrasound. From the third day of the menstrual cycle, recombinant follicle-stimulating hormone (FSH, Gonal-F 75; Merck Serono S.p.A, 70026-Modugno, Italy) was used to oversaturate ovaries. The daily dose was determined by a gynecologist based on the patient’s specifications and history. The growth of follicles was controlled by the ultrasound method. When the size of at least three follicles attained 18 mm or more, human chorionic gonadotropin (hCG) was administered at 1000 IU; 36 hours later, the ovaries were punctured by a physician and through vaginal ultrasonography. Each follicle was meticulously aspirated into a single tube. The aspiration of each follicle was conducted individually, and the FF comprising the oocyte was gathered.
Body mass index (BMI) was recorded, and fasting blood samples were obtained from each participant two times: Once prior to treatment on the fifth day following the menstrual cycle and again on the day of ovum pick-up (OPU) during the ICSI cycle. After collecting peripheral blood samples, they were centrifuged at 1500g for 10 minutes at room temperature using a centrifuge (EBA20, Hettich, Tuttlingen, Germany). The resulting serum samples were then preserved at -70 °C until further analysis. Similarly, the FF samples were immediately subjected to centrifugation at 1500 g for 10 minutes at room temperature, with the supernatants collected and stored at -70 °C until analysis.
Commercial enzyme-linked immunosorbent assay (ELISA) kits (Demeditec Diagnostics Inc., Kiel, Germany) were used to measure the concentrations of FF and serum levels of luteinizing hormone (LH; in mIU/mL; catalogue no. DE1289), total testosterone (TT; in ng/mL; catalogue no. DE1559), fasting insulin in mIU/L (catalogue no. DE2935), and estradiol (E2; in pg/mL; catalogue no. DE2693). The concentration of anti-Mullerian hormone (AMH; in ng/mL) in the FF was measured using a second-generation enzyme immunoassay (AMH-EIA kit; catalogue no. A92269C; Immunotech Beckman Coulter, Brea, CA, USA), according to the instructions of the manufacturer.
The level of malondialdehyde (MDA; in mM), a naturally occurring byproduct of lipid peroxidation, was assessed in the FF using the thiobarbituric acid (TBA) colorimetric method and a TBA-reactive substances (TBARS) assay kit (catalogue no. KA1381; Abnova Corp., Taiwan). Prior to the measurement of AMH (1:20) in FF, dilutions were carried out using the phosphate-buffered serum (PBS) buffer based on the calibration range.
ICSI and culture of oocytes
Oocyte retrieval was conducted through ultrasound-guided transvaginal aspiration utilizing a single-lumen needle (Reproline Medical GmbH, Rheinbach, Germany). The cumulus cells surrounding all oocytes were removed by a 30-second exposure to a solution of 20 IU/mL hyaluronidase (ART-4007A; SAGE BioPharma, Pasadena, CA, USA) in a HEPES-buffered medium. This procedure was subsequently followed by thorough washing with HEPES-buffered human tubal fluid (HTF) that contained 5 mg/mL of human serum albumin (ART-3001; SAGE BioPharma, England) and mechanical pipetting. Mature oocytes at the metaphase II stage (MII) were identified by observing the first polar body using a stereomicroscope (Olympus, Tokyo, Japan). The most effective oocytes that successfully extruded the first polar body were selected for ICSI. Prior to the injection, an organized sperm suspension was added to a 50-mL droplet of polyvinylpolypyrrolidone (PVP; ART-4006-A; SAGE BioPharma, England). Four hours after oocyte retrieval, a spermatozoa with good parameters exhibiting normal morphology was immobilized and utilized for insemination of the oocyte. The inseminated oocytes were subsequently placed in fertilization medium (ART-1520; SAGE BioPharma, England) and were covered by mineral oil (Reproline Medical GmbH, Rheinbach, Germany). The next day, fertilization was assessed, and fertilized eggs were placed in an equilibrated cleavage medium (ART-1526; SAGE BioPharma, England) if two pronuclei (2PN) were seen. Using an embryo transfer catheter (Labotect, Göttingen, Germany), embryos were transferred 48-72 hours following oocyte retrieval. For each patient, a maximum of four embryos were transferred. Starting from the embryo transfer day and continuing until the pregnancy test, 100 mg progesterone injections were given intramuscularly every day to provide luteal phase support (Gestone, London, UK). Serum β-hCG measurements on days 16–17 post-transfer and the finding of a gestational sac and heartbeat during ultrasound examinations seven weeks following the embryo transfer were used to confirm pregnancy.
Evaluation of oocyte morphology, fertilization, and embryo quality
Using an Olympus inverted microscope (IX71) with a Hoffmann modulation contrast system at a magnification of 400x, the first polar body detected just before the ICSI operation was used to measure the nuclear maturation of oocytes. The MII oocyte morphological scoring system (MOMS) and the grading criteria developed by Rienzi et al. who considered granularity (including the size and distribution of granules, whether they were clustered or homogeneously distributed, and their location within the oocyte), the size of the perivitelline space, and the arrangement of organelles (including vacuoles and endoplasmic reticulum) were used to examine the oocytes’ morphology [18]. Oocytes were divided into three groups according to these morphological evaluations: (a) normal oocytes, (b) oocytes with extracytoplasmic abnormalities (like a dark zona pellucida, a large perivitelline space, and a fragmented polar body), and (c) oocytes with intracytoplasmic abnormalities (like vacuolation, structural deformities, cytoplasmic fragments, and dark or granular cytoplasm). The meiotic spindle was visualized using polarized light technology (CRi PolScope Technology, London, UK).
By looking for two different pronuclei, fertilization results were assessed 12-16 hours after ICSI. During 24-36 hours following fertilization, cleavage was observed. On the third day after insemination, the embryos’ quality was evaluated and categorized as follows: (a) Uneven blastomeres with less than 30% fragmentation found in grade II, (b) symmetric blastomeres with no fragmentation found in grade I, and (c) unequal blastomeres with more than 30% fragmentation found in grade III [19].
Statistical analysis
The Kolmogorov–Smirnov test confirmed that continuous variables had a normal distribution. The Mean±SD was used to describe the data. Chi-square test and one-way ANOVA were used for data analysis, followed by post hoc comparisons using Tukey’s test and Dunnett’s T3 test. P≤0.05 was regarded as statistically significant. Pearson’s correlation test was used to evaluate the relationship between the variables. Statistical analyses were conducted in SPSS software, version 13.
Results
Clinical and demographic characteristics
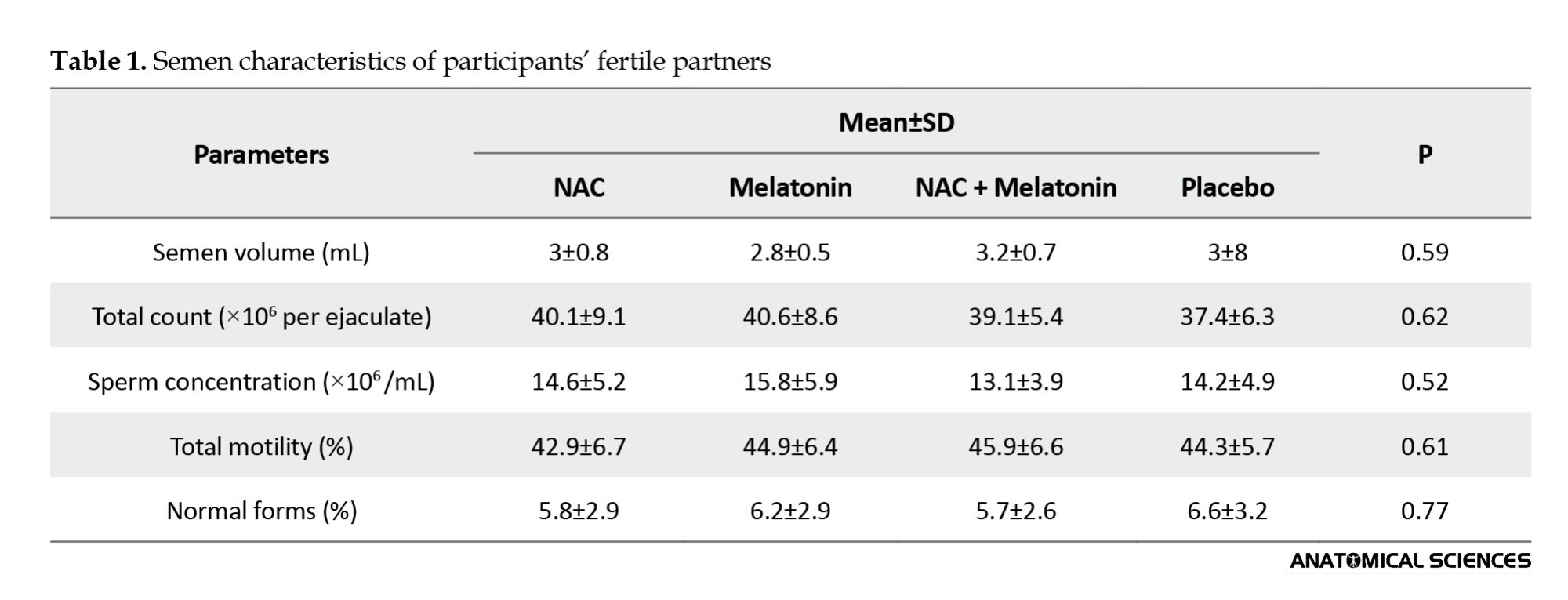
The results showed that BMI, oligomenorrhea, amenorrhea, length of marriage, duration of infertility, age, hirsutism, and levels of insulin, LH, FSH, TT, and E2 were not significantly different among the four groups prior to therapy. Also, there was no significant difference between the groups in the partner’s sperm parameters (Table 1).
Comparison based on hormonal parameters of the FF
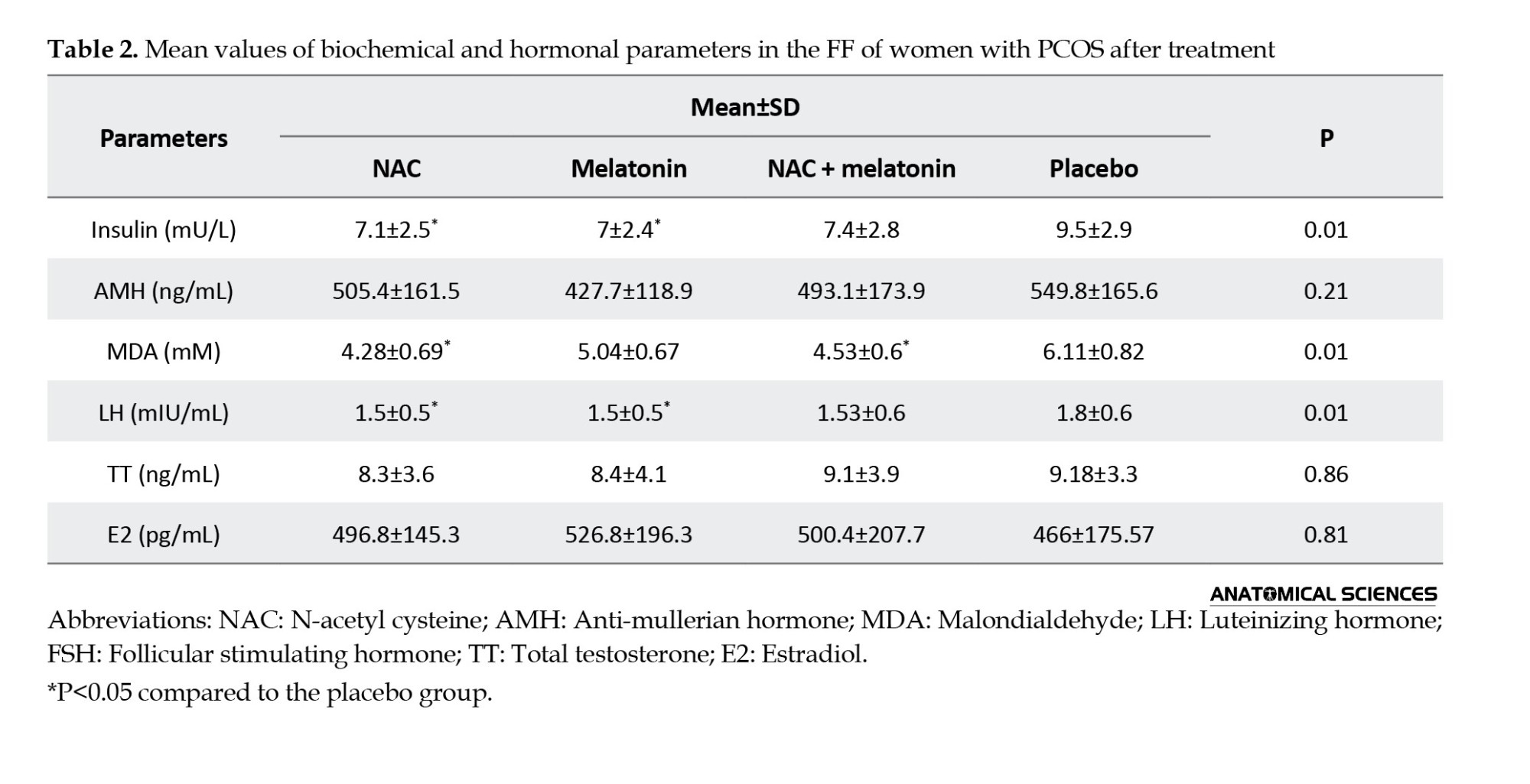
As seen in Table 2, insulin and LH levels in FF were significantly lower in the melatonin (7±2.4 and 1.5±0.5, respectively) and NAC (7.1±2.5 and 1.5±0.5, respectively) groups than in the placebo group (9.5±2.9 and 1.8±0.6, respectively) (P=0.01). However, TT, E2, and AMH concentrations in FF were not significantly different between treatment groups and the placebo group (P>0.05). The MDA level was significantly lower in the NAC (4.28±0.69) and melatonin + NAC (4.53±0.6) groups than in the melatonin (5.04±0.67) and placebo (6.11±0.82) groups (Table 2). The difference between treatment groups and the placebo group in MDA level was statistically significant (P=0.01).
Comparison based on oocyte and embryo morphology
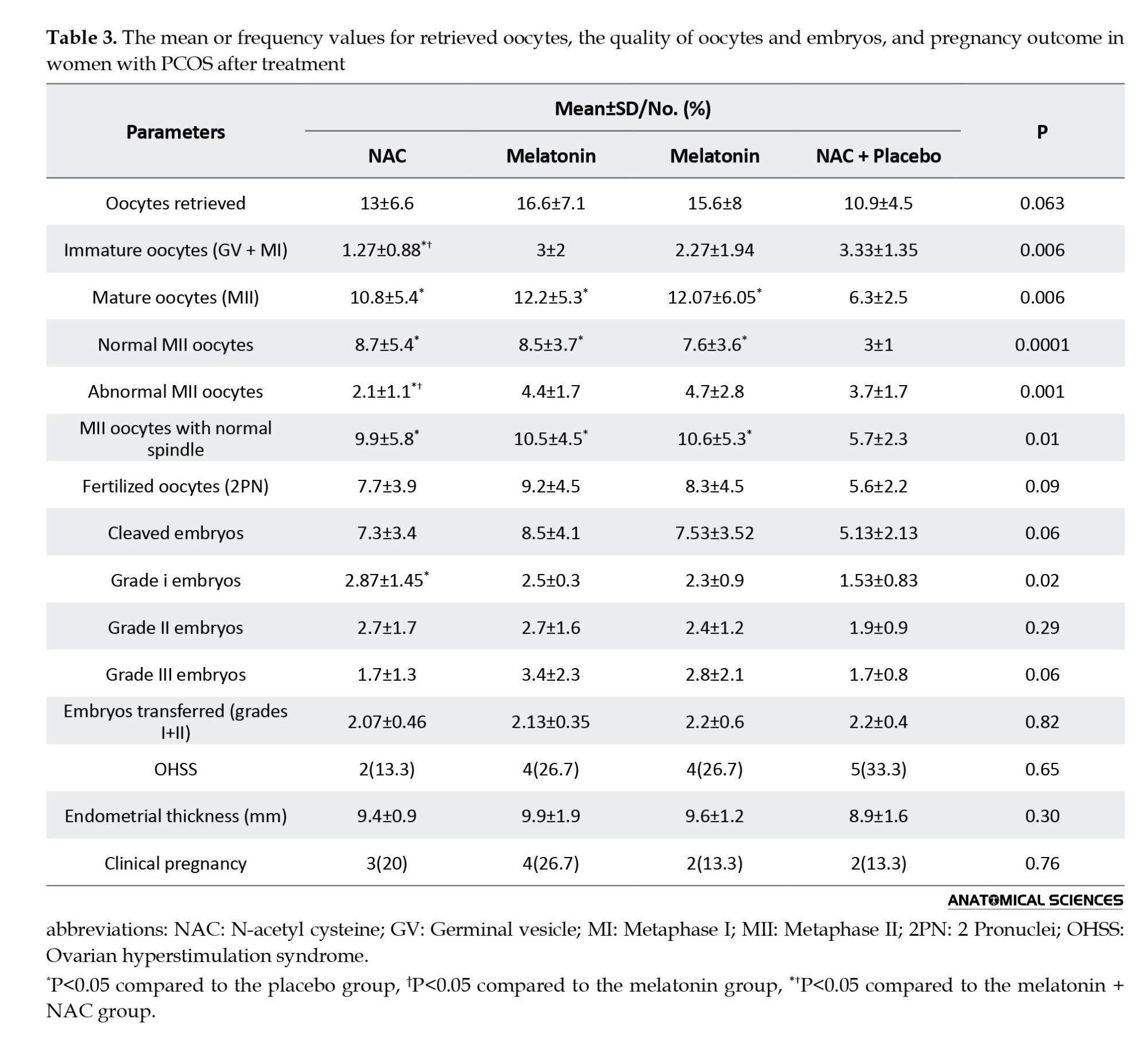
As seen in Table 3, the number of oocytes retrieved from three treatment groups (NAC: 13±6.6, melatonin: 16.6±7.1, melatonin + NAC: 15.6±8) was not significantly different compared to the placebo group (10.9±4.5) (P=0.06). The NAC group had the lowest number of immature oocytes (in two metaphase I and germinal vesicle stages) and abnormal mature MII oocytes with values of 1.27±0.88 and 2.1±1.1, respectively, among the treatment groups compared to the placebo (3.33±1.35 and 3.7±1.7, respectively). In these factors, the difference between treatment groups and the placebo group was significant (P=0.006 and P=0.001, respectively). The number of normal MII oocytes was significantly greater in the melatonin (8.7±5.4) and NAC (8.5±3.7) treatment groups compared to the placebo group (3±1) (P<0.001). Furthermore, all treatment groups showed a significantly higher number of MII oocytes with normal meiotic spindles around the second polar body (NAC: 9.9±5.8, melatonin: 10.5±4.5, melatonin+NAC: 10.6±5.3) compared to the placebo group (5.7±2.3) (P=0.01). The number of fertilized MII oocytes (P=0.09) and cleaved embryos (P=0.06) was not different significantly among the groups. Only the NAC group showed a significantly higher number of high-quality (grade I) embryos on day three compared to the placebo group (2.87±1.45 vs. 1.53±0.83; P=0.02). The melatonin (2.5±0.3) and melatonin+NAC (2.3±0.9) groups did not exhibit such a significant increase compared to the placebo group (P>0.05). There was no significant difference among the four groups in the number of grade II (P=0.29) and III (P=0.06) embryos and the number of transferred embryos (grade I+II) (P=0.82). Additionally, there was no significant difference in the incidence of endometrial thickness (P=0.30) and ovarian hyperstimulation syndrome (OHSS) (P=0.65) among the four groups (Table 3). There was a strong negative correlation between the fertilization rate and MDA level in FF (r=-0.28; P=0.02) and between the number of mature oocytes with meiotic spindle and MDA level in FF (r=-0.294; P=0.02).
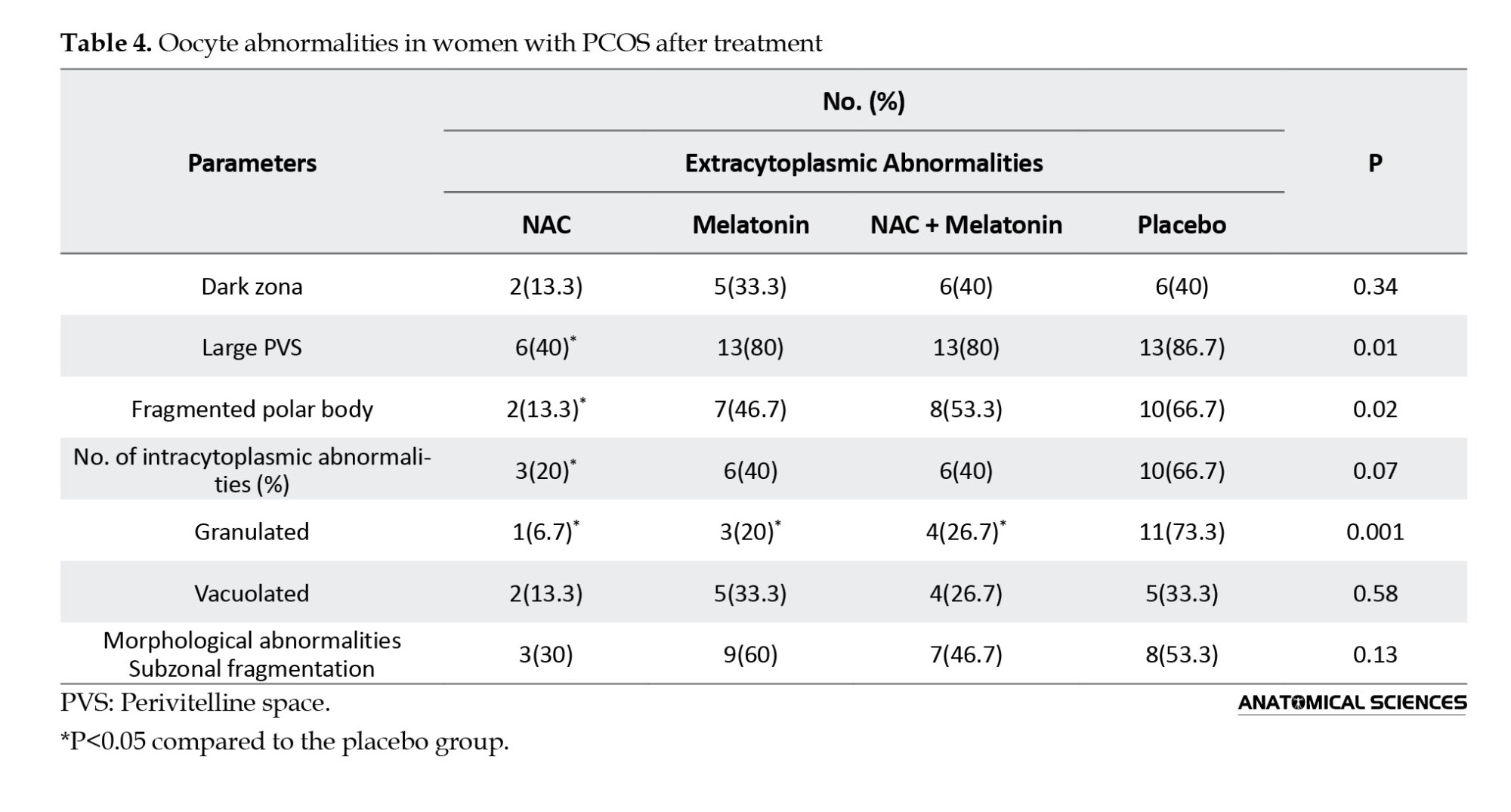
The study of oocyte intracytoplasmic and extracytoplasmic abnormalities revealed that the NAC group had a significantly lower polar body fragmentation rate (13.3%, P=0.02) and lower large perivitelline space (40%) compared to the placebo group (66.7% and 86.7%, respectively) (P=0.01). The difference in polar body fragmentation rate and large perivitelline space of the melatonin and melatonin+NAC groups was not statistically significant compared to the placebo group (P>0.05). Compared to the placebo group (73.3%), the prevalence of vacuolated oocytes was significantly lower in the NAC (6.7%), melatonin (20%), and melatonin + NAC (26.7%) groups (P=0.001). Compared to the placebo group, there was a tendency for the treatment groups to have decreased rates of dark zona, subzonal fragmentation, and morphological abnormalities; however, these changes were not statistically significant (Table 4).
Discussion
The results of this study showed that the NAC group of women with PCOS had a significantly lower number of immature and abnormal oocytes but a higher number of grade I embryos compared to the placebo group. Furthermore, the NAC and melatonin+NAC groups had significantly lower MDA level, and the melatonin and NAC groups had significantly lower insulin and LH levels compared to the placebo group.
Several studies have been conducted in recent years to reduce the problems associated with PCOS. A primary goal of the proposed treatments is to alleviate oxidative stress through the administration of various antioxidants. Women with PCOS often exhibit persistently elevated levels of LH, which is believed to adversely affect oocyte maturation and embryo quality, potentially hindering implantation and leading to a higher incidence of spontaneous abortions [4]. Our results indicated that administration of NAC and melatonin improves the oocyte quality and implantation rates [20], which are associated with elevated estrogen levels. Additionally, significant declines in oocyte quality, reduced maturation, lower fertilization rate, compromised embryo quality, decreased pregnancy rate, and increased miscarriage rate are linked to high LH concentrations, elevated reactive oxygen species in FF, and diminished total antioxidant capacity [21].
Melatonin is found in many organs, but when it is administered regularly, it accumulates in the ovary, eye, and pineal gland [22]. Mature follicles have larger intrafollicular melatonin concentrations than tiny atretic follicles [23]. As a powerful scavenger of free radicals and a complete antioxidant, melatonin is essential for controlling the transcription of genes encoding antioxidant enzymes [24]. In the study by Öztürk et al. [25], rats’ livers exhibited elevated superoxide dismutase (SOD) activity after receiving 10 mg/kg of melatonin for seven days. Similarly, Liu and Ng [26] reported that, after injection of melatonin at a single dose of 5 mg/kg, there was an increase in SOD activity in the rats’ kidneys, liver, and brain. Moreover, it has been shown that melatonin affects the expression of antioxidant enzymes. Antolín et al. [27] were the first to observe that exogenous administration of melatonin (500 µg/kg) led to elevated mRNA levels in copper/zinc SOD and manganese SOD. Some studies showed that melatonin affected the production of sex steroids at various stages of ovarian follicular maturation [9, 28]. A 100 mM dose of melatonin was shown to enhance the production of progesterone and androgens in mouse preantral follicles after a 12-day incubation period [9]. Elevated level of melatonin in FF are critical for follicle development, ovulation, and the quality of oocytes, while low concentration of melatonin may contribute to anovulation, suboptimal oocyte quality in PCOS, and failures in follicular maturation [28].
Animal studies have shown that NAC is not teratogenic and mutated, with no serious side effects [11]. In 2007, Badawy et al. [29] investigated the effect of clomiphene citrate (CC), NAC, and their combination in PCOS patients, and observed that ovulation was significantly improved in the group received NAC plus CC. They suggested NAC can be used as a supplement to the CC, because NAC is well tolerated by the body and is also cost-effective. Because of its anti-apoptotic and antioxidant qualities, Cheraghi et al. demonstrated that NAC reduces insulin, LH, and leptin levels in FF, improving oocyte maturity and quality and fetal development while lowering the quantity of immature oocytes [30]. According to Javanmanesh et al.’s study in 2015 [31], patients who take NAC in addition to CC have higher levels of progesterone, ovulation, and fertility. Using the extended agonist protocol, Elgindy et al. found that 1200 mg of NAC during ICSI cycles did not significantly increase the number of grade I embryos or the rate of fertilization and pregnancy [13]. Recent findings indicate that mice subjected to a two-month treatment with NAC demonstrated better embryo development and an increase in the number and quality of oocytes. The elevation in telomerase activity and telomere length attributed to NAC can be due to a reduction in follicle atresia, thereby ensuring the quality of oocytes [32].
The findings of this study demonstrated that NAC’s antioxidant and anti-apoptotic qualities significantly reduced the levels of insulin, LH, and MDA in the FF of women with PCOS. In addition to improving embryo development, it lowers the frequency of immature oocytes and increases the maturation and quality of oocytes. These results support the findings of other studies [29, 32, 33]. Furthermore, our results suggested that there may be no synergistic effect when NAC and melatonin are administered concurrently, or the doses of NAC or melatonin might be inadequate.
Our findings are consistent with those of Rajani et al. [34], who found that a higher number of oocytes with a normal meiotic spindle and high MDA level resulted in lower fertilization rate and lower-quality embryos. Our research showed a strong negative association between fertilization rate and MDA level in FF and between the number of mature oocytes with a meiotic spindle and MDA level in FF. Limitations of this study include quantity of patients needed, the time needed for patients to get on the cycle, lack of cooperation of patients to enter the study, and lack of funding.
Conclusion
When administered alone to women with PCOS, melatonin and NAC have positive effects on oocyte and embryo quality; however, their combination has no positive synergistic effects. Using NAC along with anti-insulin resistance drugs, such as metformin, may have positive effects on oocyte and embryo quality.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC.1394.27418). All participants declared informed consent.
Funding
This research was funded by Iran University of Medical Sciences, Tehran, Iran (Grant No.: 27418).
Authors' contributions
Supervision, project administration, and funding acquisition: Reza Shiraz and Sajed Khaledii; Data collection, investigation, resources, writing: Sajed Khaledi ; Review & editing: Mehrdad Ghorbanlou; Software, validation, formal analysis: Maryam Najafpour Pitka and Zahra Sadat Hoseini.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the participant and the staff of the IVF Unit at Shahid Akbar Abadi Hospital in Tehran, for their cooperation and assistance in this study.
References
With a 15% incidence in couples, infertility is a complex disorder caused by several factors [1]. One of the main causes of infertility in women is polycystic ovary syndrome (PCOS), affecting about 10-15% of women before menopause [2]. PCOS is also linked to irregular ovulation, insulin resistance, and elevated androgen levels, which can result in metabolic complications, including diabetes and cardiovascular diseases [3]. Oocyte quality is a critical factor in the success of assisted reproductive technologies. During ovulation, women with PCOS, typically exhibit a higher quantity of oocytes. However, these oocytes frequently possess inferior quality, resulting in diminished rates of fertilization, cleavage, and implantation [4]. Chronic inflammation, increased oxidative stress, and the subsequent increased cell apoptosis lead to follicular growth disorders and an increased number of atretic follicles in PCOS patients [5, 6]. An imbalance between prooxidants and antioxidants ultimately increases the apoptosis of granulosa cells in PCOS patients and causes symptoms of the disease [7]. Oxidative stress and apoptosis can be reduced by using antioxidants.
Melatonin (N-acetyl-5-methoxytryptamine) is primarily produced by the pineal gland at night. However, it can also be found in other organs, including retina, extraorbital lacrimal gland, Harderian gland, gastrointestinal tract’s enterochromaffin cells, and gut and bone marrow cells. This hormone plays a crucial role in regulating various central and peripheral functions associated with circadian rhythms and reproductive processes [8, 9]. As an indirect antioxidant, Melatonin influences biological processes indirectly by promoting antioxidative enzymes and suppressing prooxidative enzymes. In addition, it serves as a potent direct scavenger of free radicals [10].
N-acetylcysteine (NAC), as a mucolytic agent derived from the amino acid L-cysteine, is a strong antioxidant, destroying free radicals by being converted into metabolites which stimulate glutathione production. Insulin receptors and insulin secretion are affected by NAC. It also increases stored glucose [11]. NAC is an antioxidant, anti-inflammatory, and anti-apoptotic agent, contributing to maintaining vascular integrity and exhibiting immunological effects [12]. It may represent a promising strategy for enhancing or stimulating ovulation in women experiencing chronic anovulation, such as those with PCOS.
In this study, we aim to examine the impacts of NAC, melatonin, and their combination on the quality of embryos and oocytes, in addition to the biochemical parameters present in the follicular fluid (FF), in women with PCOS candidate for intracytoplasmic sperm injection (ICSI). We hypothesized that the concurrent use of melatonin and NAC can enhance insulin sensitivity and promote ovulation in these women.
Materials and Methods
Study design and participants
This is a randomized placebo-controlled clinical trial conducted on 60 Iranian women aged 25-35 referred to the infertility clinic of Shahid Akbar Abadi Hospital in Tehran, Iran, from May 2015 to August 2017, who were candidates for ICSI due to PCOS-related infertility. The sample size was determined based on previous studies [13, 14]. Patients were required to meet the diagnostic criteria for PCOS defined by the 2003 Rotterdam Consensus Workshop [15], which is based on the presence of at least two of the following criteria: Chronic oligo- or an-ovulation, clinical or biochemical signs of hyperandrogenism, and polycystic ovaries as observed through ultrasound examination. Exclusion criteria were hypersensitivity to melatonin or NAC, infertility unrelated to anovulation, male infertility, pelvic organic pathologies, congenital adrenal hyperplasia, thyroid dysfunction, Cushing’s syndrome, hyperprolactinaemia, androgen-secreting tumors, diabetes mellitus, the use of medications that affect carbohydrate metabolism and hormonal analogues except for progesterone in two months prior to inclusion, or severe liver or kidney dysfunction. Semen specimens from male partners were evaluated in accordance with World Health Organization (WHO) guidelines [16], and women with partners having irregular semen characteristics were not included in the study. Lack of cooperation, unwillingness to continue the study, and failure of ovulation induction were the reasons for excluding eight women from the study. Finally, 52 patients underwent analysis (Figure 1). All participants were asked not to make any modifications in their regular bodily activity and food plan, and not to use any new medications during the study.

Treatment protocol
The patients were divided into four groups of 15, (i) placebo group, receiving an oral rehydration solution (Razi, Tehran, Iran) three times daily; (ii) melatonin group, receiving melatonin (KAL, Park City, UT, USA) 3 mg per day at 10:00 PM; (ii) NAC group, receiving 600 mg NAC (Batch no. 6N5483; Holzkrichen, Bavaria, Germany) three times daily; and (iv) melatonin+NAC group, receiving the same doses of melatonin and NAC simultaneously. A midwife administered the medication to the patients, ensuring that both the patients and the physicians remained unaware of the treatment protocols. The dosage and duration of NAC treatment were determined based on prior research [14, 17]. All treatments were administered for 40 days, commencing from the fifth day of previous menstrual cycle until the day designated for oocyte aspiration. In addition, all participants were prescribed oral contraceptive pills (OCP) for 21 days, which initiated concurrently with the administration of the placebo, melatonin, NAC, or the melatonin+NAC on the fifth day following menstruation, prior to the start of the treatment cycle. During the study, patients did not show any side effects except one in the melatonin group who had a headache.
Sample collection
The Long protocol [15] was used to stimulate ovulation. Among the analogues of gonadotropin-releasing hormone (GnRH), triptorelin (Decapeptyl; Ferring GmbH, Wittland 11, D-24109 Kiel, Germany) or an antagonist of the GnRH, cetrorelix (Cetrotide; Kantstrasse 2, D-33790 Halle, Germany) was used according to the physician’s opinion. Triptorelin 0.14 mg for 14 days and cetrorelix 0.25 mg/day were injected to observe at least one follicle or more with a size of 14 mm by transvaginal ultrasound. From the third day of the menstrual cycle, recombinant follicle-stimulating hormone (FSH, Gonal-F 75; Merck Serono S.p.A, 70026-Modugno, Italy) was used to oversaturate ovaries. The daily dose was determined by a gynecologist based on the patient’s specifications and history. The growth of follicles was controlled by the ultrasound method. When the size of at least three follicles attained 18 mm or more, human chorionic gonadotropin (hCG) was administered at 1000 IU; 36 hours later, the ovaries were punctured by a physician and through vaginal ultrasonography. Each follicle was meticulously aspirated into a single tube. The aspiration of each follicle was conducted individually, and the FF comprising the oocyte was gathered.
Body mass index (BMI) was recorded, and fasting blood samples were obtained from each participant two times: Once prior to treatment on the fifth day following the menstrual cycle and again on the day of ovum pick-up (OPU) during the ICSI cycle. After collecting peripheral blood samples, they were centrifuged at 1500g for 10 minutes at room temperature using a centrifuge (EBA20, Hettich, Tuttlingen, Germany). The resulting serum samples were then preserved at -70 °C until further analysis. Similarly, the FF samples were immediately subjected to centrifugation at 1500 g for 10 minutes at room temperature, with the supernatants collected and stored at -70 °C until analysis.
Commercial enzyme-linked immunosorbent assay (ELISA) kits (Demeditec Diagnostics Inc., Kiel, Germany) were used to measure the concentrations of FF and serum levels of luteinizing hormone (LH; in mIU/mL; catalogue no. DE1289), total testosterone (TT; in ng/mL; catalogue no. DE1559), fasting insulin in mIU/L (catalogue no. DE2935), and estradiol (E2; in pg/mL; catalogue no. DE2693). The concentration of anti-Mullerian hormone (AMH; in ng/mL) in the FF was measured using a second-generation enzyme immunoassay (AMH-EIA kit; catalogue no. A92269C; Immunotech Beckman Coulter, Brea, CA, USA), according to the instructions of the manufacturer.
The level of malondialdehyde (MDA; in mM), a naturally occurring byproduct of lipid peroxidation, was assessed in the FF using the thiobarbituric acid (TBA) colorimetric method and a TBA-reactive substances (TBARS) assay kit (catalogue no. KA1381; Abnova Corp., Taiwan). Prior to the measurement of AMH (1:20) in FF, dilutions were carried out using the phosphate-buffered serum (PBS) buffer based on the calibration range.
ICSI and culture of oocytes
Oocyte retrieval was conducted through ultrasound-guided transvaginal aspiration utilizing a single-lumen needle (Reproline Medical GmbH, Rheinbach, Germany). The cumulus cells surrounding all oocytes were removed by a 30-second exposure to a solution of 20 IU/mL hyaluronidase (ART-4007A; SAGE BioPharma, Pasadena, CA, USA) in a HEPES-buffered medium. This procedure was subsequently followed by thorough washing with HEPES-buffered human tubal fluid (HTF) that contained 5 mg/mL of human serum albumin (ART-3001; SAGE BioPharma, England) and mechanical pipetting. Mature oocytes at the metaphase II stage (MII) were identified by observing the first polar body using a stereomicroscope (Olympus, Tokyo, Japan). The most effective oocytes that successfully extruded the first polar body were selected for ICSI. Prior to the injection, an organized sperm suspension was added to a 50-mL droplet of polyvinylpolypyrrolidone (PVP; ART-4006-A; SAGE BioPharma, England). Four hours after oocyte retrieval, a spermatozoa with good parameters exhibiting normal morphology was immobilized and utilized for insemination of the oocyte. The inseminated oocytes were subsequently placed in fertilization medium (ART-1520; SAGE BioPharma, England) and were covered by mineral oil (Reproline Medical GmbH, Rheinbach, Germany). The next day, fertilization was assessed, and fertilized eggs were placed in an equilibrated cleavage medium (ART-1526; SAGE BioPharma, England) if two pronuclei (2PN) were seen. Using an embryo transfer catheter (Labotect, Göttingen, Germany), embryos were transferred 48-72 hours following oocyte retrieval. For each patient, a maximum of four embryos were transferred. Starting from the embryo transfer day and continuing until the pregnancy test, 100 mg progesterone injections were given intramuscularly every day to provide luteal phase support (Gestone, London, UK). Serum β-hCG measurements on days 16–17 post-transfer and the finding of a gestational sac and heartbeat during ultrasound examinations seven weeks following the embryo transfer were used to confirm pregnancy.
Evaluation of oocyte morphology, fertilization, and embryo quality
Using an Olympus inverted microscope (IX71) with a Hoffmann modulation contrast system at a magnification of 400x, the first polar body detected just before the ICSI operation was used to measure the nuclear maturation of oocytes. The MII oocyte morphological scoring system (MOMS) and the grading criteria developed by Rienzi et al. who considered granularity (including the size and distribution of granules, whether they were clustered or homogeneously distributed, and their location within the oocyte), the size of the perivitelline space, and the arrangement of organelles (including vacuoles and endoplasmic reticulum) were used to examine the oocytes’ morphology [18]. Oocytes were divided into three groups according to these morphological evaluations: (a) normal oocytes, (b) oocytes with extracytoplasmic abnormalities (like a dark zona pellucida, a large perivitelline space, and a fragmented polar body), and (c) oocytes with intracytoplasmic abnormalities (like vacuolation, structural deformities, cytoplasmic fragments, and dark or granular cytoplasm). The meiotic spindle was visualized using polarized light technology (CRi PolScope Technology, London, UK).
By looking for two different pronuclei, fertilization results were assessed 12-16 hours after ICSI. During 24-36 hours following fertilization, cleavage was observed. On the third day after insemination, the embryos’ quality was evaluated and categorized as follows: (a) Uneven blastomeres with less than 30% fragmentation found in grade II, (b) symmetric blastomeres with no fragmentation found in grade I, and (c) unequal blastomeres with more than 30% fragmentation found in grade III [19].
Statistical analysis
The Kolmogorov–Smirnov test confirmed that continuous variables had a normal distribution. The Mean±SD was used to describe the data. Chi-square test and one-way ANOVA were used for data analysis, followed by post hoc comparisons using Tukey’s test and Dunnett’s T3 test. P≤0.05 was regarded as statistically significant. Pearson’s correlation test was used to evaluate the relationship between the variables. Statistical analyses were conducted in SPSS software, version 13.
Results
Clinical and demographic characteristics
The results showed that BMI, oligomenorrhea, amenorrhea, length of marriage, duration of infertility, age, hirsutism, and levels of insulin, LH, FSH, TT, and E2 were not significantly different among the four groups prior to therapy. Also, there was no significant difference between the groups in the partner’s sperm parameters (Table 1).

Comparison based on hormonal parameters of the FF
As seen in Table 2, insulin and LH levels in FF were significantly lower in the melatonin (7±2.4 and 1.5±0.5, respectively) and NAC (7.1±2.5 and 1.5±0.5, respectively) groups than in the placebo group (9.5±2.9 and 1.8±0.6, respectively) (P=0.01). However, TT, E2, and AMH concentrations in FF were not significantly different between treatment groups and the placebo group (P>0.05). The MDA level was significantly lower in the NAC (4.28±0.69) and melatonin + NAC (4.53±0.6) groups than in the melatonin (5.04±0.67) and placebo (6.11±0.82) groups (Table 2). The difference between treatment groups and the placebo group in MDA level was statistically significant (P=0.01).

Comparison based on oocyte and embryo morphology
As seen in Table 3, the number of oocytes retrieved from three treatment groups (NAC: 13±6.6, melatonin: 16.6±7.1, melatonin + NAC: 15.6±8) was not significantly different compared to the placebo group (10.9±4.5) (P=0.06). The NAC group had the lowest number of immature oocytes (in two metaphase I and germinal vesicle stages) and abnormal mature MII oocytes with values of 1.27±0.88 and 2.1±1.1, respectively, among the treatment groups compared to the placebo (3.33±1.35 and 3.7±1.7, respectively). In these factors, the difference between treatment groups and the placebo group was significant (P=0.006 and P=0.001, respectively). The number of normal MII oocytes was significantly greater in the melatonin (8.7±5.4) and NAC (8.5±3.7) treatment groups compared to the placebo group (3±1) (P<0.001). Furthermore, all treatment groups showed a significantly higher number of MII oocytes with normal meiotic spindles around the second polar body (NAC: 9.9±5.8, melatonin: 10.5±4.5, melatonin+NAC: 10.6±5.3) compared to the placebo group (5.7±2.3) (P=0.01). The number of fertilized MII oocytes (P=0.09) and cleaved embryos (P=0.06) was not different significantly among the groups. Only the NAC group showed a significantly higher number of high-quality (grade I) embryos on day three compared to the placebo group (2.87±1.45 vs. 1.53±0.83; P=0.02). The melatonin (2.5±0.3) and melatonin+NAC (2.3±0.9) groups did not exhibit such a significant increase compared to the placebo group (P>0.05). There was no significant difference among the four groups in the number of grade II (P=0.29) and III (P=0.06) embryos and the number of transferred embryos (grade I+II) (P=0.82). Additionally, there was no significant difference in the incidence of endometrial thickness (P=0.30) and ovarian hyperstimulation syndrome (OHSS) (P=0.65) among the four groups (Table 3). There was a strong negative correlation between the fertilization rate and MDA level in FF (r=-0.28; P=0.02) and between the number of mature oocytes with meiotic spindle and MDA level in FF (r=-0.294; P=0.02).

The study of oocyte intracytoplasmic and extracytoplasmic abnormalities revealed that the NAC group had a significantly lower polar body fragmentation rate (13.3%, P=0.02) and lower large perivitelline space (40%) compared to the placebo group (66.7% and 86.7%, respectively) (P=0.01). The difference in polar body fragmentation rate and large perivitelline space of the melatonin and melatonin+NAC groups was not statistically significant compared to the placebo group (P>0.05). Compared to the placebo group (73.3%), the prevalence of vacuolated oocytes was significantly lower in the NAC (6.7%), melatonin (20%), and melatonin + NAC (26.7%) groups (P=0.001). Compared to the placebo group, there was a tendency for the treatment groups to have decreased rates of dark zona, subzonal fragmentation, and morphological abnormalities; however, these changes were not statistically significant (Table 4).

Discussion
The results of this study showed that the NAC group of women with PCOS had a significantly lower number of immature and abnormal oocytes but a higher number of grade I embryos compared to the placebo group. Furthermore, the NAC and melatonin+NAC groups had significantly lower MDA level, and the melatonin and NAC groups had significantly lower insulin and LH levels compared to the placebo group.
Several studies have been conducted in recent years to reduce the problems associated with PCOS. A primary goal of the proposed treatments is to alleviate oxidative stress through the administration of various antioxidants. Women with PCOS often exhibit persistently elevated levels of LH, which is believed to adversely affect oocyte maturation and embryo quality, potentially hindering implantation and leading to a higher incidence of spontaneous abortions [4]. Our results indicated that administration of NAC and melatonin improves the oocyte quality and implantation rates [20], which are associated with elevated estrogen levels. Additionally, significant declines in oocyte quality, reduced maturation, lower fertilization rate, compromised embryo quality, decreased pregnancy rate, and increased miscarriage rate are linked to high LH concentrations, elevated reactive oxygen species in FF, and diminished total antioxidant capacity [21].
Melatonin is found in many organs, but when it is administered regularly, it accumulates in the ovary, eye, and pineal gland [22]. Mature follicles have larger intrafollicular melatonin concentrations than tiny atretic follicles [23]. As a powerful scavenger of free radicals and a complete antioxidant, melatonin is essential for controlling the transcription of genes encoding antioxidant enzymes [24]. In the study by Öztürk et al. [25], rats’ livers exhibited elevated superoxide dismutase (SOD) activity after receiving 10 mg/kg of melatonin for seven days. Similarly, Liu and Ng [26] reported that, after injection of melatonin at a single dose of 5 mg/kg, there was an increase in SOD activity in the rats’ kidneys, liver, and brain. Moreover, it has been shown that melatonin affects the expression of antioxidant enzymes. Antolín et al. [27] were the first to observe that exogenous administration of melatonin (500 µg/kg) led to elevated mRNA levels in copper/zinc SOD and manganese SOD. Some studies showed that melatonin affected the production of sex steroids at various stages of ovarian follicular maturation [9, 28]. A 100 mM dose of melatonin was shown to enhance the production of progesterone and androgens in mouse preantral follicles after a 12-day incubation period [9]. Elevated level of melatonin in FF are critical for follicle development, ovulation, and the quality of oocytes, while low concentration of melatonin may contribute to anovulation, suboptimal oocyte quality in PCOS, and failures in follicular maturation [28].
Animal studies have shown that NAC is not teratogenic and mutated, with no serious side effects [11]. In 2007, Badawy et al. [29] investigated the effect of clomiphene citrate (CC), NAC, and their combination in PCOS patients, and observed that ovulation was significantly improved in the group received NAC plus CC. They suggested NAC can be used as a supplement to the CC, because NAC is well tolerated by the body and is also cost-effective. Because of its anti-apoptotic and antioxidant qualities, Cheraghi et al. demonstrated that NAC reduces insulin, LH, and leptin levels in FF, improving oocyte maturity and quality and fetal development while lowering the quantity of immature oocytes [30]. According to Javanmanesh et al.’s study in 2015 [31], patients who take NAC in addition to CC have higher levels of progesterone, ovulation, and fertility. Using the extended agonist protocol, Elgindy et al. found that 1200 mg of NAC during ICSI cycles did not significantly increase the number of grade I embryos or the rate of fertilization and pregnancy [13]. Recent findings indicate that mice subjected to a two-month treatment with NAC demonstrated better embryo development and an increase in the number and quality of oocytes. The elevation in telomerase activity and telomere length attributed to NAC can be due to a reduction in follicle atresia, thereby ensuring the quality of oocytes [32].
The findings of this study demonstrated that NAC’s antioxidant and anti-apoptotic qualities significantly reduced the levels of insulin, LH, and MDA in the FF of women with PCOS. In addition to improving embryo development, it lowers the frequency of immature oocytes and increases the maturation and quality of oocytes. These results support the findings of other studies [29, 32, 33]. Furthermore, our results suggested that there may be no synergistic effect when NAC and melatonin are administered concurrently, or the doses of NAC or melatonin might be inadequate.
Our findings are consistent with those of Rajani et al. [34], who found that a higher number of oocytes with a normal meiotic spindle and high MDA level resulted in lower fertilization rate and lower-quality embryos. Our research showed a strong negative association between fertilization rate and MDA level in FF and between the number of mature oocytes with a meiotic spindle and MDA level in FF. Limitations of this study include quantity of patients needed, the time needed for patients to get on the cycle, lack of cooperation of patients to enter the study, and lack of funding.
Conclusion
When administered alone to women with PCOS, melatonin and NAC have positive effects on oocyte and embryo quality; however, their combination has no positive synergistic effects. Using NAC along with anti-insulin resistance drugs, such as metformin, may have positive effects on oocyte and embryo quality.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Iran University of Medical Sciences, Tehran, Iran (Code: IR.IUMS.REC.1394.27418). All participants declared informed consent.
Funding
This research was funded by Iran University of Medical Sciences, Tehran, Iran (Grant No.: 27418).
Authors' contributions
Supervision, project administration, and funding acquisition: Reza Shiraz and Sajed Khaledii; Data collection, investigation, resources, writing: Sajed Khaledi ; Review & editing: Mehrdad Ghorbanlou; Software, validation, formal analysis: Maryam Najafpour Pitka and Zahra Sadat Hoseini.
Conflict of interest
The authors declared no conflict of interest.
Acknowledgments
The authors would like to thank the participant and the staff of the IVF Unit at Shahid Akbar Abadi Hospital in Tehran, for their cooperation and assistance in this study.
References
- Knuttinen MG, Jajko R, Scoccia B. Fluoroscopic tubal recanalization in tubal factor related infertility. Seminars in Interventional Radiology. 2014; 31(3):269-71. [DOI:10.1055/s-0034-1382797] [PMID]
- Diamanti-Kandarakis E. Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Reviews in Molecular Medicine. 2008; 10:e3. [DOI:10.1017/S1462399408000598] [PMID]
- Thakker D, Raval A, Patel I, Walia R. N‐acetylcysteine for polycystic ovary syndrome: A systematic review and meta‐analysis of randomized controlled clinical trials. Obstetrics and Gynecology International. 2015; 2015(1):817849. [DOI:10.1155/2015/817849] [PMID]
- Kumar P, Nawani N, Malhotra N, Malhotra J, Patil M, Jayakrishnan K, et al. Assisted reproduction in polycystic ovarian disease: A multicentric trial in India. Journal of Human Reproductive Sciences. 2013; 6(1):49-53. [DOI:10.4103/0974-1208.112382] [PMID]
- Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. International Journal of Women’s Health. 2011; 3:25-35. [DOI:10.2147/IJWH.S11304] [PMID]
- Karuputhula NB, Chattopadhyay R, Chakravarty B, Chaudhury K. Oxidative status in granulosa cells of infertile women undergoing IVF. Systems Biology in Reproductive Medicine. 2013; 59(2):91-8. [DOI:10.3109/19396368.2012.743197] [PMID]
- Jain P, Jain M, Haldar C, Singh TB, Jain S. Melatonin and its correlation with testosterone in polycystic ovarian syndrome. Journal of Human Reproductive Sciences. 2013; 6(4):253-8. [DOI:10.4103/0974-1208.126295] [PMID]
- Tamura H, Takasaki A, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, et al. Melatonin as a free radical scavenger in the ovarian follicle. Endocrine Journal. 2013; 60(1):1-3. [DOI:10.1507/endocrj.EJ18-0435] [PMID]
- Adriaens I, Jacquet P, Cortvrindt R, Janssen K, Smitz J. Melatonin has dose-dependent effects on folliculogenesis, oocyte maturation capacity and steroidogenesis. Toxicology. 2006; 228(2-3):333-43. [DOI:10.1016/j.tox.2006.09.018] [PMID]
- Kilic ÜK. Pharmacological utility of melatonin in reducing oxidative cellular and molecular damage. Sciences. Polish Journal of. 2004; 56:159-70. [Link]
- Kumarapeli V, Seneviratne RD, Wijeyaratne CN, Yapa RM, Dodampahala SH. A simple screening approach for assessing community prevalence and phenotype of polycystic ovary syndrome in a semiurban population in Sri Lanka. American Journal of Epidemiology. 2008; 168(3):321-8. [DOI:10.1093/aje/kwn137] [PMID]
- De Flora S, Izzotti A, D’Agostini F, Balansky RM. Mechanisms of N-acetylcysteine in the prevention of DNA damage and cancer, with special reference to smoking-related end-points. Carcinogenesis. 2001; 22(7):999-1013. [DOI:10.1093/carcin/22.7.999] [PMID]
- Elgindy EA, El-Huseiny AM, Mostafa MI, Gaballah AM, Ahmed TA. N-acetyl cysteine: Could it be an effective adjuvant therapy in ICSI cycles? A preliminary study. Reproductive Biomedicine Online. 2010; 20(6):789-96. [DOI:10.1016/j.rbmo.2010.03.001] [PMID]
- Elnashar A, Fahmy M, Mansour A, Ibrahim K. N-acetyl cysteine vs. metformin in treatment of clomiphene citrate-resistant polycystic ovary syndrome: A prospective randomized controlled study. Fertility and Sterility. 2007; 88(2):406-9. [DOI:10.1016/j.fertnstert.2006.11.173] [PMID]
- Pan ML, Chen LR, Tsao HM, Chen KH. Relationship between polycystic ovarian syndrome and subsequent gestational diabetes mellitus: A nationwide population-based study. PloS One. 2015; 10(10):e0140544. [DOI:10.1371/journal.pone.0140544] [PMID]
- Cooper TG, Noonan E, Von Eckardstein S, Auger J, Baker HG, Behre HM, et al. World Health Organization reference values for human semen characteristics. Human Reproduction Update. 2010; 16(3):231-45. [DOI:10.1093/humupd/dmp048] [PMID]
- Fulghesu AM, Ciampelli M, Muzj G, Belosi C, Selvaggi L, Ayala GF, Lanzone A. N-acetyl-cysteine treatment improves insulin sensitivity in women with polycystic ovary syndrome. Fertility and Sterility. 2002; 77(6):1128-35. [DOI:10.1016/S0015-0282(02)03133-3] [PMID]
- Rienzi L, Balaban B, Ebner T, Mandelbaum J. The oocyte. Human Reproduction. 2012; 27(suppl_1):i2-1. [DOI:10.1093/humrep/des200] [PMID]
- Brezinova J, Oborna I, Svobodova M, Fingerova H. Evaluation of day one embryo quality and IVF outcome-a comparison of two scoring systems. Reproductive Biology and Endocrinology. 2009; 7:1-6. [DOI:10.1186/1477-7827-7-9] [PMID]
- Ashkenazi J, Farhi J, Orvieto R, Homburg R, Dekel A, Feldberg D, et al. Polycystic ovary syndrome patients as oocyte donors: The effect of ovarian stimulation protocol on the implantation rate of the recipient. Fertility and Sterility. 1995; 64(3):564-7. [DOI:10.1016/S0015-0282(16)57793-0] [PMID]
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: A review. Reproductive Biology and Endocrinology. 2012; 10:1-31. [DOI:10.1186/1477-7827-10-49] [PMID]
- Wurtman RJ, Axelrod J, Potter LT. The uptake of H3-melatonin in endocrine and nervous tissues and the effects of constant light exposure. Journal of Pharmacology and Experimental Therapeutics. 1964; 143:314-8. [DOI:10.1016/S0022-3565(25)26732-5] [PMID]
- Nakamura Y, Tamura H, Takayama H, Kato H. Increased endogenous level of melatonin in preovulatory human follicles does not directly influence progesterone production. Fertility and Sterility. 2003; 80(4):1012-6. [DOI:10.1016/S0015-0282(03)01008-2] [PMID]
- Leon J, Acuña‐Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. Journal of Pineal Research. 2005; 38(1):1-9. [DOI:10.1111/j.1600-079X.2004.00181.x] [PMID]
- Öztürk G, Coşkun Ş, Erbaş D, Hasanoglu E. The effect of melatonin on liver superoxide dismutase activity, serum nitrate and thyroid hormone levels. The Japanese Journal of Physiology. 2000; 50(1):149-53. [DOI:10.2170/jjphysiol.50.149] [PMID]
- Liu F, Ng TB. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochemistry and Cell Biology. 2000; 78(4):447-53. [DOI:10.1139/o00-018] [PMID]
- Antolín IS, Rodríguez C, Sáinz RM, Mayo JC, Uría H, Kotler ML, et al. Neurohormone melatonin prevents cell damage: Effect On gene expression for antioxidant enzymes. The FASEB Journal. 1996; 10(8):882-90. [DOI:10.1096/fasebj.10.8.8666165] [PMID]
- Tamura H, Nakamura Y, Korkmaz A, Manchester LC, Tan DX, Sugino N, et al. Melatonin and the ovary: Physiological and pathophysiological implications. Fertility and Sterility. 2009; 92(1):328-43. [DOI:10.1016/j.fertnstert.2008.05.016] [PMID]
- Badawy A, State O, Abdelgawad S. N-Acetyl cysteine and clomiphene citrate for induction of ovulation in polycystic ovary syndrome: A cross-over trial. Acta Obstetricia et Gynecologica Scandinavica. 2007; 86(2):218-22. [DOI:10.1080/00016340601090337] [PMID]
- Cheraghi E, Mehranjani MS, Shariatzadeh MA, Esfahani MH, Ebrahimi Z. N-Acetylcysteine improves oocyte and embryo quality in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection: An alternative to metformin. Reproduction, Fertility and Development. 2016; 28(6):723-31. [DOI:10.1071/RD14182] [PMID]
- Javanmanesh F, Kashanian M, Rahimi M, Sheikhansari N. A comparison between the effects of metformin and N-acetyl cysteine (NAC) on some metabolic and endocrine characteristics of women with polycystic ovary syndrome. Gynecological Endocrinology. 2016; 32(4):285-9. [DOI:10.3109/09513590.2015.1115974] [PMID]
- Liu J, Liu M, Ye X, Liu K, Huang J, Wang L, et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Human Reproduction. 2012; 27(5):1411-20. [DOI:10.1093/humrep/des019] [PMID]
- Oner G, Muderris II. Clinical, endocrine and metabolic effects of metformin vs N-acetyl-cysteine in women with polycystic ovary syndrome. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2011; 159(1):127-31. [DOI:10.1016/j.ejogrb.2011.07.005] [PMID]
- Rajani S, Chattopadhyay R, Goswami SK, Ghosh S, Sharma S, Chakravarty B. Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF-ET outcome. Journal of Human Reproductive Sciences. 2012; 5(2):187-93. [DOI:10.4103/0974-1208.101020] [PMID]
Type of Study: Original |
Subject:
Reproductive Biology
Received: 2018/07/3 | Accepted: 2024/09/11 | Published: 2023/08/30
Received: 2018/07/3 | Accepted: 2024/09/11 | Published: 2023/08/30
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |



