Thu, Jan 1, 2026
Volume 14, Issue 2 (Summer & Autumn 2017)
ASJ 2017, 14(2): 83-90 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Erfani Majd N, Moradi H R, Moftakhar P. The Effects of Urtica Dioica L. Root Extract on Rat Testis. ASJ 2017; 14 (2) :83-90
URL: http://anatomyjournal.ir/article-1-169-en.html
URL: http://anatomyjournal.ir/article-1-169-en.html
1- Department of Clinical Sciences, Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
2- Department of Basic Sciences, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran.
2- Department of Basic Sciences, Faculty of Veterinary Medicine, University of Tehran, Tehran, Iran.
Full-Text [PDF 602 kb]
(2044 Downloads)
| Abstract (HTML) (6126 Views)
Full-Text: (14653 Views)
1. Introduction
Stinging nettle (Urtica dioica L.) is the most important genus in the plant family Urticaceae, which includes 50 species [1]. Urtica dioica root extract is widely used for the treatment of hormonal disorders such as prostate disorders [2]. The antiproliferative [3], antiviral [4], antimicrobial, antiulcer, antioxidant, and analgesic effects [5] of U. dioica are indicated in vitro. Although aqueous and alcoholic extracts of U. dioica root contain various compounds, the active ingredients of such extracts are still unknown. Nettle effects are attributed to more than 1 class of chemical substances. Phytoestrogens, available in a group of plants, contain components with structural similarity to natural and synthetic estrogen and anti-estrogen [6]. In addition, phytoestrogens can inhibit the activity of 5-alpha-reductase and 17β-HSD enzymes [7].
Adlercreutz et al. reported that phytoestrogens compete with androgens through the inhibition of binding the substrate to aromatase enzyme [8]. The anti-estradiol and anti-estrogen effects in reproductive system may be caused by phytoestrogens and this role is played by their structural similarity to hormone estradiol [9, 10]. Estradiol in males is predominantly produced by peripheral aromatization of testicular and adrenal androgens [11]. Testosterone is secreted by testes and stimulates the sperm production [12]. The active and more potent androgen in testes is dihydrotestosterone (DHT), although testosterone is the major androgen in testes [13, 14]. Furthermore, the role of testosterone was reported as an essential factor in normal spermatogenesis of testes. The conversion process of spherical spermatids to elongated spermatids (between the stages VII and VIII of spermatogenic cycle) is stimulated by testosterone. Testosterone deficiency causes disorderliness in spermatogenesis [15].
On the other hand, U. dioica root extract is widely used for treatment of benign prostatic hyperplasia as it has anti-androgen effects, but there are no information on the histological effects of UDE on testes and spermatogenesis. The current study aimed at studying the effects of U. dioica root extract, alone or in combination with testosterone, on testicular structure and spermatogenesis.
2. Materials and Methods
Urtica dioica root extract (Barij Essence, Iran), testosterone (Nile; Egypt), almond oil (Kimiagar Toos; Iran) and testosterone measurement kit (DRG; Germany) were used in the current study.
Study design
Twenty-five adult male Wistar rats with healthy appearance and the mean weight of 290±20 g and mean age of 3.5 to 4 months were used. Rats were kept under a 12:12 hours light/dark cycle at 23–25˚C temperature. Rats were given ad libitum access to feed. Animals were adapted to environmental conditions for 1 week. Rats were divided randomly into 5 groups: G1 (the control group) kept under the environmental and nutritional conditions similar to other groups without any treatment; G2 (the testosterone group) received 10 mg/kg testosterone subcutaneously daily; G3 (the UDE group) received 50 mg/kg UDE orally (via gavage) daily; G4 (the testosterone+UDE group) received 10 mg/kg testosterone subcutaneously plus 50 mg/kg UDE orally daily; and G5 (almond oil group) received 10 mg/kg almond oil subcutaneously (almond oil was used as testosterone solvent).
The intervention lasted 42 days. Testosterone and UDE dosage were administered according to the authors’ previous studies [1, 16]. The study was adopted according to the regulations by the Iranian Veterinary Organization and manuals published by the Ethical Committee of the Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Iran.
Histomorphometric studies
Animals were weighed. Rats were sacrificed using chloroform; blood samples were collected for the assessment of testosterone using Enzyme-Linked Immune Sorbent Assay (ELISA) technique. The testes were removed carefully. The epididymis was separated from testes and then, weight and volume of the testes were recorded. Volume of testes was measured by the water displacement method [16]. Samples were fixed in 10% buffered formalin following the standard histological procedure. The 5-6-μm thickness sections were stained using Hematoxylin & Eosin (H&E). Dino-Lite digital lens and Dino Capture 2 software (AnMo Electronics Corp., New Taipei City, Taiwan) were used for the microscopic analyses. Furthermore, the histological structure of testes were studied, including epithelium thickness of seminiferous tubules, number of Sertoli and Leydig cells, seminiferous tubules diameter, and size of Leydig cells.
Spermatogenesis and spermiogenesis were studied based on Tubular Differentiation Index (TDI), Repopulation Index (RI), and Spermatogenesis Index (SI) analyses. To analyze TDI, the percentage of seminiferous tubules showing ≥4 layers of differentiated germinal cells from spermatogonia type A were prepared (i.e. intermediate or type B spermatogonia, spermatocytes, or spermatids) and the seminiferous tubules showed more than 4 layers were considered as TDI positive. RI was a ratio of type B to type A spermatogonia. SI was a ratio of seminiferous tubules containing spermatid cells to the total tubules (as SI positive) and the ratio of tubules lacking spermatids to the total tubules (as SI negative) [17].
Serum testosterone level
Blood serum was separated by centrifugation at 3000 g for 10 minutes and stored at -70°C until use. Serum testosterone was assessed using ELISA technique. Statistical data analysis: Data were expressed as mean±Standard Deviation (SD). Data were analyzed statistically with SPSS version 16. The one-way ANOVA and the post hoc Tukey were performed on the data. P<0.05 was considered as level of significance.
3. Results
Data analyses showed no significant difference in body weight and net weight of the testes among the groups. Relative weight of testes in the U. dioica extract group showed a significant increase, compared with that of the control group (P<0.05). In the current study, volume of the testes in the U. dioica extract group showed no significant increase, compared with that of the control group. Furthermore, volume of the testes in the testosterone group revealed a significant decrease, compared with that of the U. dioica extract group (P<0.05) (Table 1).
Stinging nettle (Urtica dioica L.) is the most important genus in the plant family Urticaceae, which includes 50 species [1]. Urtica dioica root extract is widely used for the treatment of hormonal disorders such as prostate disorders [2]. The antiproliferative [3], antiviral [4], antimicrobial, antiulcer, antioxidant, and analgesic effects [5] of U. dioica are indicated in vitro. Although aqueous and alcoholic extracts of U. dioica root contain various compounds, the active ingredients of such extracts are still unknown. Nettle effects are attributed to more than 1 class of chemical substances. Phytoestrogens, available in a group of plants, contain components with structural similarity to natural and synthetic estrogen and anti-estrogen [6]. In addition, phytoestrogens can inhibit the activity of 5-alpha-reductase and 17β-HSD enzymes [7].
Adlercreutz et al. reported that phytoestrogens compete with androgens through the inhibition of binding the substrate to aromatase enzyme [8]. The anti-estradiol and anti-estrogen effects in reproductive system may be caused by phytoestrogens and this role is played by their structural similarity to hormone estradiol [9, 10]. Estradiol in males is predominantly produced by peripheral aromatization of testicular and adrenal androgens [11]. Testosterone is secreted by testes and stimulates the sperm production [12]. The active and more potent androgen in testes is dihydrotestosterone (DHT), although testosterone is the major androgen in testes [13, 14]. Furthermore, the role of testosterone was reported as an essential factor in normal spermatogenesis of testes. The conversion process of spherical spermatids to elongated spermatids (between the stages VII and VIII of spermatogenic cycle) is stimulated by testosterone. Testosterone deficiency causes disorderliness in spermatogenesis [15].
On the other hand, U. dioica root extract is widely used for treatment of benign prostatic hyperplasia as it has anti-androgen effects, but there are no information on the histological effects of UDE on testes and spermatogenesis. The current study aimed at studying the effects of U. dioica root extract, alone or in combination with testosterone, on testicular structure and spermatogenesis.
2. Materials and Methods
Urtica dioica root extract (Barij Essence, Iran), testosterone (Nile; Egypt), almond oil (Kimiagar Toos; Iran) and testosterone measurement kit (DRG; Germany) were used in the current study.
Study design
Twenty-five adult male Wistar rats with healthy appearance and the mean weight of 290±20 g and mean age of 3.5 to 4 months were used. Rats were kept under a 12:12 hours light/dark cycle at 23–25˚C temperature. Rats were given ad libitum access to feed. Animals were adapted to environmental conditions for 1 week. Rats were divided randomly into 5 groups: G1 (the control group) kept under the environmental and nutritional conditions similar to other groups without any treatment; G2 (the testosterone group) received 10 mg/kg testosterone subcutaneously daily; G3 (the UDE group) received 50 mg/kg UDE orally (via gavage) daily; G4 (the testosterone+UDE group) received 10 mg/kg testosterone subcutaneously plus 50 mg/kg UDE orally daily; and G5 (almond oil group) received 10 mg/kg almond oil subcutaneously (almond oil was used as testosterone solvent).
The intervention lasted 42 days. Testosterone and UDE dosage were administered according to the authors’ previous studies [1, 16]. The study was adopted according to the regulations by the Iranian Veterinary Organization and manuals published by the Ethical Committee of the Faculty of Veterinary Medicine, Shahid Chamran University of Ahvaz, Iran.
Histomorphometric studies
Animals were weighed. Rats were sacrificed using chloroform; blood samples were collected for the assessment of testosterone using Enzyme-Linked Immune Sorbent Assay (ELISA) technique. The testes were removed carefully. The epididymis was separated from testes and then, weight and volume of the testes were recorded. Volume of testes was measured by the water displacement method [16]. Samples were fixed in 10% buffered formalin following the standard histological procedure. The 5-6-μm thickness sections were stained using Hematoxylin & Eosin (H&E). Dino-Lite digital lens and Dino Capture 2 software (AnMo Electronics Corp., New Taipei City, Taiwan) were used for the microscopic analyses. Furthermore, the histological structure of testes were studied, including epithelium thickness of seminiferous tubules, number of Sertoli and Leydig cells, seminiferous tubules diameter, and size of Leydig cells.
Spermatogenesis and spermiogenesis were studied based on Tubular Differentiation Index (TDI), Repopulation Index (RI), and Spermatogenesis Index (SI) analyses. To analyze TDI, the percentage of seminiferous tubules showing ≥4 layers of differentiated germinal cells from spermatogonia type A were prepared (i.e. intermediate or type B spermatogonia, spermatocytes, or spermatids) and the seminiferous tubules showed more than 4 layers were considered as TDI positive. RI was a ratio of type B to type A spermatogonia. SI was a ratio of seminiferous tubules containing spermatid cells to the total tubules (as SI positive) and the ratio of tubules lacking spermatids to the total tubules (as SI negative) [17].
Serum testosterone level
Blood serum was separated by centrifugation at 3000 g for 10 minutes and stored at -70°C until use. Serum testosterone was assessed using ELISA technique. Statistical data analysis: Data were expressed as mean±Standard Deviation (SD). Data were analyzed statistically with SPSS version 16. The one-way ANOVA and the post hoc Tukey were performed on the data. P<0.05 was considered as level of significance.
3. Results
Data analyses showed no significant difference in body weight and net weight of the testes among the groups. Relative weight of testes in the U. dioica extract group showed a significant increase, compared with that of the control group (P<0.05). In the current study, volume of the testes in the U. dioica extract group showed no significant increase, compared with that of the control group. Furthermore, volume of the testes in the testosterone group revealed a significant decrease, compared with that of the U. dioica extract group (P<0.05) (Table 1).
Histological studies
In the current study, testis tissue samples of the control group were composed of coiled seminiferous tubules with high density surrounded by interstitial connective tissue (blood vessels, Leydig cells, and lamina propria). The Leydig cells had an abundant, eosinophilic cytoplasm with large, round nuclei and prominent nucleoli. Small and large vacuoles were observed in cytoplasm of these cells that probably were the hormonal storage. Furthermore, the maturing elongated spermatids were attached to the cell membrane of Sertoli cells in the late stage of spermiogenes. Sertoli cells were also counted based on the accumulation place of spermatids at triangular points in seminiferous tubule.
Spermatogonia had a round nucleus located in tubules. A and B spermatogonia included euchromatin and heterochromatin nuclei, respectively. Primary spermatocytes were the largest spermatogenic cells in the tubular epithelium with vary shapes of chromatin. Other cells with small and round nuclei located in seminiferous tubules lumen were the spermatids. Furthermore, cells with elongated nuclei were the maturing spermatids (Figures 1 and 2A). In the current study, exposure of testis tissue to U. dioica root extract decreased the density of seminiferous tubules, which led to increase in the interstitial connective tissue (with Leydig cells) and degenerative changes in seminiferous tubules, compared with those of the control group (Figure 2C).
In the current study, testis tissue samples of the control group were composed of coiled seminiferous tubules with high density surrounded by interstitial connective tissue (blood vessels, Leydig cells, and lamina propria). The Leydig cells had an abundant, eosinophilic cytoplasm with large, round nuclei and prominent nucleoli. Small and large vacuoles were observed in cytoplasm of these cells that probably were the hormonal storage. Furthermore, the maturing elongated spermatids were attached to the cell membrane of Sertoli cells in the late stage of spermiogenes. Sertoli cells were also counted based on the accumulation place of spermatids at triangular points in seminiferous tubule.
Spermatogonia had a round nucleus located in tubules. A and B spermatogonia included euchromatin and heterochromatin nuclei, respectively. Primary spermatocytes were the largest spermatogenic cells in the tubular epithelium with vary shapes of chromatin. Other cells with small and round nuclei located in seminiferous tubules lumen were the spermatids. Furthermore, cells with elongated nuclei were the maturing spermatids (Figures 1 and 2A). In the current study, exposure of testis tissue to U. dioica root extract decreased the density of seminiferous tubules, which led to increase in the interstitial connective tissue (with Leydig cells) and degenerative changes in seminiferous tubules, compared with those of the control group (Figure 2C).
The degenerating seminiferous tubules were not observed in other groups. The Leydig cells were seen as clusters surrounding the blood vessels in the UDE group. The U. dioica extract receiving group showed a decrease in seminiferous tubules with ≥4 lines of differentiated cells, compared with that of the control group. Furthermore, disorders were observed in unification and adherence of spermatogenic cells in seminiferous germinal epithelium.
Vacuolar degeneration was reported in germinal epithelium of seminiferous tubules. On the contrary, histological studies on the groups receiving testosterone showed an increase in the number and density of seminiferous tubules; hence, basement membranes of seminiferous tubules were close to the basement membrane of adjacent seminiferous tubules, and interstitial connective tissue showed a decrease, compared with that of the control group. The groups receiving testosterone showed recovery and regularity of epithelial cells in the histological structure of testes as well as the increased activity of seminiferous tubules (Figure 2B).
Microscopic studies
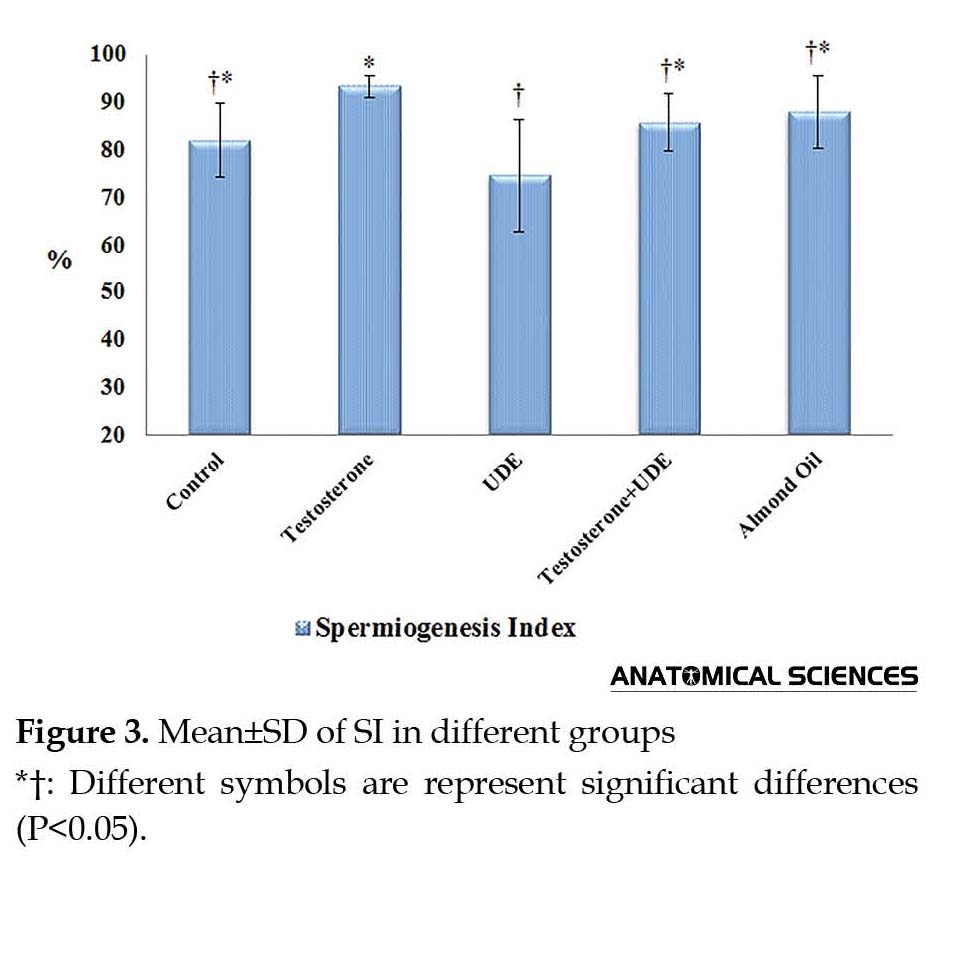
As shown in Figure 3, SI showed a significant decrease in the group receiving U. dioica root extract, compared with that of the control group (P<0.05). On the contrary, receiving testosterone led to a significant increase in SI, compared with that of the UDE group (P<0.05). SI showed no significant increase in the testosterone group, compared with that of the control group (Figure 3). TDI showed a significant increase in the testosterone group, compared with the UDE group (P<0.05). TDI showed no significant increase in the testosterone group, compared with that of the control group. In addition, no significant decrease was observed in TDI in the UDE group, compared with that of the control group (Figure 4). RI revealed a significant increase in the testosterone group (64.5%±8.5%), compared with those of the control, UDE, and almond oil groups (P<0.05) (Figure 5).
Vacuolar degeneration was reported in germinal epithelium of seminiferous tubules. On the contrary, histological studies on the groups receiving testosterone showed an increase in the number and density of seminiferous tubules; hence, basement membranes of seminiferous tubules were close to the basement membrane of adjacent seminiferous tubules, and interstitial connective tissue showed a decrease, compared with that of the control group. The groups receiving testosterone showed recovery and regularity of epithelial cells in the histological structure of testes as well as the increased activity of seminiferous tubules (Figure 2B).
Microscopic studies
As shown in Figure 3, SI showed a significant decrease in the group receiving U. dioica root extract, compared with that of the control group (P<0.05). On the contrary, receiving testosterone led to a significant increase in SI, compared with that of the UDE group (P<0.05). SI showed no significant increase in the testosterone group, compared with that of the control group (Figure 3). TDI showed a significant increase in the testosterone group, compared with the UDE group (P<0.05). TDI showed no significant increase in the testosterone group, compared with that of the control group. In addition, no significant decrease was observed in TDI in the UDE group, compared with that of the control group (Figure 4). RI revealed a significant increase in the testosterone group (64.5%±8.5%), compared with those of the control, UDE, and almond oil groups (P<0.05) (Figure 5).
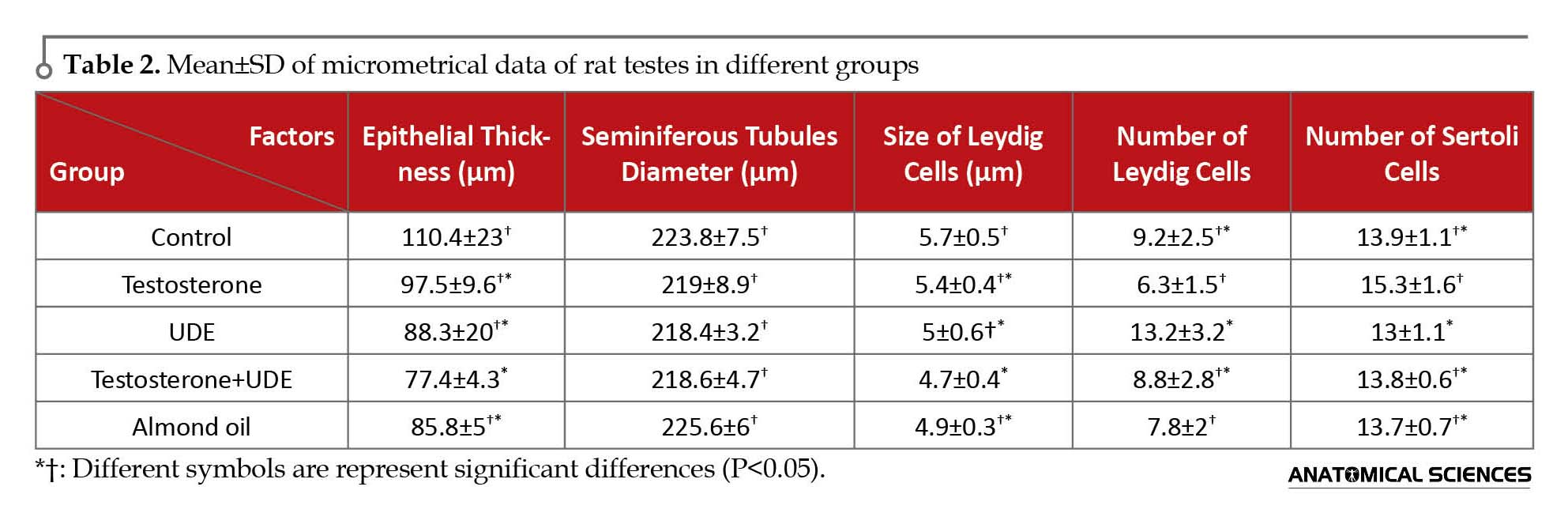
The maximum thickness of germinal epithelium of seminiferous tubules in the control group is shown in Table 2. There was no significant decrease in the epithelial thickness among the groups receiving U. dioica root extract, compared with those of other groups. Statistical analysis of data showed no significant difference in seminiferous tubules diameter as well as size of Leydig cells of testes among the groups.
Microscopic results showed that testosterone can increase the number of Sertoli cells of seminiferous tubules. Number of Sertoli cells in the testosterone group showed a significant increase, compared with that of the UDE group (P<0.05). On the contrary, receiving U. dioica root extract caused no significant decrease in the number of Sertoli cells, compared with that of the control group. Furthermore, number of Leydig cells in the group receiving U. dioica root extract showed a significant increase, compared with those of the testosterone and almond oil groups (P<0.05). Number of Leydig cells in the UDE group showed no significant increase, compared with that of the control group (Table 2).
Serum testosterone assessment
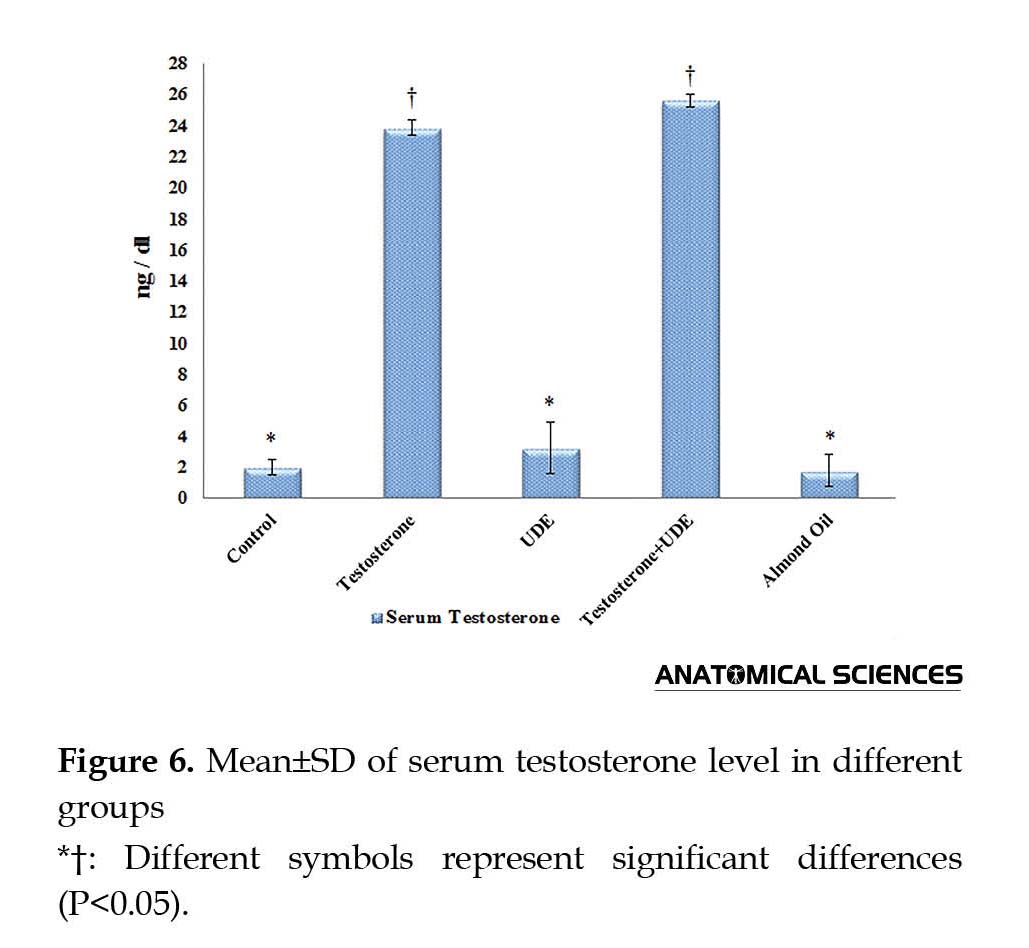
As shown in Figure 6, serum testosterone showed no significant increase in the UDE group (3.21±1.69 ng/dL), compared with that of the control group (1.97±0.47 ng/dL). Serum testosterone showed a significant increase in the groups receiving testosterone, compared with those of the other groups (P<0.05). Furthermore, serum testosterone assessments showed insignificant increase in the U. dioica root extract plus testosterone (25.59±0.41 ng/dL) group, compared with that of the testosterone group (23.83±0.47 ng/dL).
Serum testosterone assessment
As shown in Figure 6, serum testosterone showed no significant increase in the UDE group (3.21±1.69 ng/dL), compared with that of the control group (1.97±0.47 ng/dL). Serum testosterone showed a significant increase in the groups receiving testosterone, compared with those of the other groups (P<0.05). Furthermore, serum testosterone assessments showed insignificant increase in the U. dioica root extract plus testosterone (25.59±0.41 ng/dL) group, compared with that of the testosterone group (23.83±0.47 ng/dL).
4. Discussion
Carlsen et al. warned about the decline in sperm quality and quantity in healthy adult males worldwide (to less than 50%), which indicates the decline of fertility in male populations [18]. Reduction of testes weight, seminiferous tubules diameter, and histological structure of testes by phytoestrogens was reported in different studies [19, 20, 21]. Results of the present study showed that U. dioica root extract (widely used for the treatment of benign prostatic hyperplasia) had significant effects on the rat testes structure.
Administration of 50 mg/kg UDE showed a significant increase in relative weight and volume of rat testes. This increase could be due to the increased connective tissue and Leydig cells in the UDE group. Sertoli cells and spermatogenesis decreased in the UDE groups. Furthermore, seminiferous tubules diameter showed no significant changes in the UDE groups that was consistent with the study by Fritz et al. They stated that genistein not significantly changed the diameter of seminiferous tubules in the rat testes [22].
Lygans are among UDE components, which interferences the binding of androgens to Sex Hormone Binding Globulin (SHBG) [23]. Blood testosterone transfers to the target tissue by binding to SHBG [24]. Testosterone converts to the active form, DHT, in testes by 5-alpha-reductase enzyme [14]. Urtica dioica root extract is the inhibitor of 5-alpha-reductase enzyme; hence, it prevents the conversion of testosterone to DHT. Therefore, U. dioica root extract probably affects testes structure, cell proliferation, and spermatogenesis through reducing DHT level.
Positive effects of U. dioica root extract in reducing the symptoms of enlarged prostate reported in many studies. Histological and histometrical degenerative changes on prostate epithelial cells were induced by the oral administration of U. dioica root extract, which may be attributed to its antiandrogen properties. Lignans, sterols, flavonoids and polysaccharides, lecithin, and fatty acids in U. dioica root are responsible for pharmacological effects (antiandrogen role) [16].
Administration of testosterone along with UDE improved Spermatogenesis Index (SI, TDI, and RI) and testicular structure, comparison with those of the control group. According to the results of the current study, UDE may have anti-testosterone effect. The testis volume depends on the function of Leydig cells, production of sperm, interstitial fluid, and the fluid secreted by Sertoli cells [12]. In the present study, testes volume reduced in the testosterone groups, which were consistent with the results of aforementioned researches. But, relative and net weight of the testes shows an insignificant difference in the treatment groups. Magee stated that testis weight of young rats not changed significantly following the administration of genistein [25]. On the contrary, Nagao et al. demonstrated that the oral administration of genistein (dose-dependent) increased the testis weight of rats [26].
It should be noted that genistein is one of the most common components of phytoestrogen [11]. Furthermore, similar researches reported that the relative and net weights of testis, volume of testis, epithelium height, and number of Sertoli cells decreased following the use of testosterone [27, 28, 29]; it was in contradiction with the results of the current study. Mitchem et al. evaluated testosterone, DHT, and estradiol effects on hamster testes. They reported increase in the number of spermatogonia cells by testosterone, DHT, and estradiol hormones [30]; thus, the results were consistent with those of the present study. It was shown that higher serum levels of testosterone van increase spermatogenesis in seminiferous tubules (especially in surrounding seminiferous tubules, near to the capsule of testis) [4].
It is necessary to explain that consistent and inconsistent reports conflicting the present study results can be attributed to differences in animal species, duration of study, dosage, and method of the administration of hormones. Zirkin et al. stated that higher levels of testosterone can gradually lead to better survival of spermatogenesis and testis weight in male rats [31]. Leydig cells constituted about 20% of adult testis volume [24] and in the present study, their number increased in the testosterone group; hence, it seems that reduction of testis volume in the testosterone group is attributed to the decrease of testes interstitial cells (Leydig).
It was reported that the number of spermatogonia, spermatocytes, and Sertoli cells in hamster testes increased by the administration of DHT [30]. In the present study, most of the Sertoli cells were seen in the testosterone group. The mechanism of steroids effect on Sertoli and germ cells are still unknown. However, one of these 2 modes is more probable: proliferation and increasing the number of Sertoli and germ cells supported through an independent pathway of Follicle-Stimulating Hormone (FSH) or stimulation of the production of FSH from pituitary. To confirm the 2 mechanisms, it was reported that both β-androgen and estrogen receptors are found in Sertoli cells in rat testis [13, 30, 32].
In the current study, serum testosterone showed a significant increase in the testosterone+UDE group, compared with those of the control and testosterone groups. Considering the current study results, UDE may prevent the conversion of testosterone to DHT (by the inhibition of 5-alpha-reductase enzyme). As a result, the testosterone levels increased in the UDE group. Thus, it seems that the mechanisms of UDE are similar to that of finasteride (widely used for the treatment of benign prostatic hyperplasia) [33].
Another probable mechanism is the estrogenic compound of UDE, which has estrogenic effects on the target tissue [34]. It was reported that estrogen stimulates apoptosis in testis germ cells in Syrian hamsters [35]. In the current study, U. dioica root extract caused the degeneration of seminiferous tubules in addition to decrease in spermatogenesis. In conclusion, results of the current study showed that U. dioica root extract may have antitestosterone effects. Furthermore, U. dioica root extract may be impaired spermatogenesis and spermiogenesis; therefore, the authors suggest U. dioica root extract to use with caution.
Acknowledgments
The authors wish to express their gratitude to the research council of Shahid Chamran University of Ahvaz for their financial support.
Conflict of Interest
The authors declared no conflicts of interest.
References
Carlsen et al. warned about the decline in sperm quality and quantity in healthy adult males worldwide (to less than 50%), which indicates the decline of fertility in male populations [18]. Reduction of testes weight, seminiferous tubules diameter, and histological structure of testes by phytoestrogens was reported in different studies [19, 20, 21]. Results of the present study showed that U. dioica root extract (widely used for the treatment of benign prostatic hyperplasia) had significant effects on the rat testes structure.
Administration of 50 mg/kg UDE showed a significant increase in relative weight and volume of rat testes. This increase could be due to the increased connective tissue and Leydig cells in the UDE group. Sertoli cells and spermatogenesis decreased in the UDE groups. Furthermore, seminiferous tubules diameter showed no significant changes in the UDE groups that was consistent with the study by Fritz et al. They stated that genistein not significantly changed the diameter of seminiferous tubules in the rat testes [22].
Lygans are among UDE components, which interferences the binding of androgens to Sex Hormone Binding Globulin (SHBG) [23]. Blood testosterone transfers to the target tissue by binding to SHBG [24]. Testosterone converts to the active form, DHT, in testes by 5-alpha-reductase enzyme [14]. Urtica dioica root extract is the inhibitor of 5-alpha-reductase enzyme; hence, it prevents the conversion of testosterone to DHT. Therefore, U. dioica root extract probably affects testes structure, cell proliferation, and spermatogenesis through reducing DHT level.
Positive effects of U. dioica root extract in reducing the symptoms of enlarged prostate reported in many studies. Histological and histometrical degenerative changes on prostate epithelial cells were induced by the oral administration of U. dioica root extract, which may be attributed to its antiandrogen properties. Lignans, sterols, flavonoids and polysaccharides, lecithin, and fatty acids in U. dioica root are responsible for pharmacological effects (antiandrogen role) [16].
Administration of testosterone along with UDE improved Spermatogenesis Index (SI, TDI, and RI) and testicular structure, comparison with those of the control group. According to the results of the current study, UDE may have anti-testosterone effect. The testis volume depends on the function of Leydig cells, production of sperm, interstitial fluid, and the fluid secreted by Sertoli cells [12]. In the present study, testes volume reduced in the testosterone groups, which were consistent with the results of aforementioned researches. But, relative and net weight of the testes shows an insignificant difference in the treatment groups. Magee stated that testis weight of young rats not changed significantly following the administration of genistein [25]. On the contrary, Nagao et al. demonstrated that the oral administration of genistein (dose-dependent) increased the testis weight of rats [26].
It should be noted that genistein is one of the most common components of phytoestrogen [11]. Furthermore, similar researches reported that the relative and net weights of testis, volume of testis, epithelium height, and number of Sertoli cells decreased following the use of testosterone [27, 28, 29]; it was in contradiction with the results of the current study. Mitchem et al. evaluated testosterone, DHT, and estradiol effects on hamster testes. They reported increase in the number of spermatogonia cells by testosterone, DHT, and estradiol hormones [30]; thus, the results were consistent with those of the present study. It was shown that higher serum levels of testosterone van increase spermatogenesis in seminiferous tubules (especially in surrounding seminiferous tubules, near to the capsule of testis) [4].
It is necessary to explain that consistent and inconsistent reports conflicting the present study results can be attributed to differences in animal species, duration of study, dosage, and method of the administration of hormones. Zirkin et al. stated that higher levels of testosterone can gradually lead to better survival of spermatogenesis and testis weight in male rats [31]. Leydig cells constituted about 20% of adult testis volume [24] and in the present study, their number increased in the testosterone group; hence, it seems that reduction of testis volume in the testosterone group is attributed to the decrease of testes interstitial cells (Leydig).
It was reported that the number of spermatogonia, spermatocytes, and Sertoli cells in hamster testes increased by the administration of DHT [30]. In the present study, most of the Sertoli cells were seen in the testosterone group. The mechanism of steroids effect on Sertoli and germ cells are still unknown. However, one of these 2 modes is more probable: proliferation and increasing the number of Sertoli and germ cells supported through an independent pathway of Follicle-Stimulating Hormone (FSH) or stimulation of the production of FSH from pituitary. To confirm the 2 mechanisms, it was reported that both β-androgen and estrogen receptors are found in Sertoli cells in rat testis [13, 30, 32].
In the current study, serum testosterone showed a significant increase in the testosterone+UDE group, compared with those of the control and testosterone groups. Considering the current study results, UDE may prevent the conversion of testosterone to DHT (by the inhibition of 5-alpha-reductase enzyme). As a result, the testosterone levels increased in the UDE group. Thus, it seems that the mechanisms of UDE are similar to that of finasteride (widely used for the treatment of benign prostatic hyperplasia) [33].
Another probable mechanism is the estrogenic compound of UDE, which has estrogenic effects on the target tissue [34]. It was reported that estrogen stimulates apoptosis in testis germ cells in Syrian hamsters [35]. In the current study, U. dioica root extract caused the degeneration of seminiferous tubules in addition to decrease in spermatogenesis. In conclusion, results of the current study showed that U. dioica root extract may have antitestosterone effects. Furthermore, U. dioica root extract may be impaired spermatogenesis and spermiogenesis; therefore, the authors suggest U. dioica root extract to use with caution.
Acknowledgments
The authors wish to express their gratitude to the research council of Shahid Chamran University of Ahvaz for their financial support.
Conflict of Interest
The authors declared no conflicts of interest.
References
- Mohammady T, Erfanimajd N, Morovvati H, Najafzadeh, H. Evaluation of concurrent administration of testosterone and nettle extract on prostate gland of rat. Global Veterinaria. 2011; 7(2):153-7.
- Durak I, Biri H, Devrim E, Sözen S, Avcı A. Aqueous extract of Urtica dioica makes significant inhibition on adenosine deaminase activity in prostate tissue from patients with prostate cancer. Cancer Biology & Therapy. 2004; 3(9):855-7. doi: 10.4161/cbt.3.9.1038
- Konrad L, Müller HH, Lenz C, Laubinger H, Aumüller G, Lichius JJ. Antiproliferative effect on human prostate cancer cells by a stinging nettle root (Urtica dioica) extract. Planta Medica. 2000; 66(1):44-7. doi: 10.1055/s-2000-11117
- Morse HC, Horike N, Rowley MJ, Heller CG. Testosterone concentrations in testes of normal men: effects of testosterone propionate administration. The Journal of Clinical Endocrinology & Metabolism. 1973; 37(6):882-6. doi: 10.1210/jcem-37-6-882
- Gülçin I, Küfrevioglu Öİ, Oktay M, Büyükokuroglu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). Journal of Ethnopharmacology. 2004; 90(2-3):205-15. doi: 10.1016/j.jep.2003.09.028
- Kurzer MS, Xu X. Dietary phytoestrogens. Annual Review of Nutrition. 1997; 17(1):353-81. doi: 10.1146/annurev.nutr.17.1.353
- Evans B. Inhibition of 5α-reductase and 17β-hydroxysteroid dehydrogenase in genital skin fibroblasts by dietary lignans and isoflavonoids. Journal of Endocrinology. 1995; 147(2):295-302. doi: 10.1677/joe.0.1470295
- Adlercreutz H, Bannwart C, Wähälä K, Mäkelä T, Brunow G, Hase T, et al. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. The Journal of Steroid Biochemistry and Molecular Biology. 1993; 44(2):147-53. doi: 10.1016/0960-0760(93)90022-o
- Maskarinec S. The effect of phytoestrogens on hot flashes. Nutrition Bytes. 2003; 9(2):1-9.
- Setchell KD, Cassidy A. Dietary isoflavones: Biological effects and relevance to human health. The Journal of Nutrition. 1999; 129(3):758S-67S. PMID: 10082786
- Opalka M, Kaminska B, Ciereszko R, Dusza L. Genistein affects testosterone secretion by Leydig cells in roosters (Gallus gallus domesticus). Reproductive Biology. 2004; 4(2):185-93. PMID: 15297892
- Murdakai T, Buraimoh AA, Kwanashie HO. Histological observations of the testis of Wistar rats following the oral administration of cotecxin (dihyroartemisinin). International Journal of Animal and Veterinary Advances. 2011; 3(5):402-6.
- O’donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocrine Reviews. 2001; 22(3):289-318. doi: 10.1210/er.22.3.289
- Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990; 126(2):1165-72. doi: 10.1210/endo-126-2-1165
- Saad F, Yassin AA, Haider A, Gooren L. Effects of testosterone on the lower urinary tract go beyond the prostate: New insights, new treatment options. Arab Journal of Urology. 2011; 9(2):147-52. doi: 10.1016/j.aju.2011.06.003
- Moradi HR, Majd NE, Esmaeilzadeh S, Tabatabaei SR. The histological and histometrical effects of Urtica dioica extract on rat’s prostate hyperplasia. Veterinary Research Forum. 2015; 6(1):23-9.
- Adibmoradi M, Morovvati H, Moradi HR, Sheybani MT, Amoli JS, Mazaheri Nezhad Fard R. Protective effects of wheat sprout on testicular toxicity in male rats exposed to lead. Reproductive System & Sexual Disorders. 2015; 4(4):1-9. doi: 10.4172/2161-038x.1000156
- Carlsen E, Giwercman A, Keiding N, Skakkebæk NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992; 305(6854):609-13. doi: 10.1136/bmj.305.6854.609
- Adachi T, Ono Y, Koh KB, Takashima K, Tainaka H, Matsuno Y, et al. Long-term alteration of gene expression without morphological change in testis after neonatal exposure to genistein in mice: toxicogenomic analysis using cDNA microarray. Food and Chemical Toxicology. 2004; 42(3):445-52. doi: 10.1016/j.fct.2003.10.012
- Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, et al. Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: Evidence for stimulatory effects of low estrogen levels. Endocrinology. 2000; 141(10):3898-907. doi: 10.1210/endo.141.10.7723
- McClain RM, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food and Chemical Toxicology. 2006; 44(1):56-80. doi: 10.1016/j.fct.2005.05.021
- Fritz WA, Cotroneo MS, Wang J, Eltoum IE, Lamartiniere CA. Dietary diethylstilbestrol but not genistein adversely affects rat testicular development. The Journal of Nutrition. 2003; 133(7):2287-93.
- Schöttner M, Ganßer D, Spiteller G. Lignans from the roots of Urtica dioica and their metabolites bind to human sex hormone binding globulin (SHBG). Planta Medica. 1997; 63(6):529-32. doi: 10.1055/s-2006-957756
- Hall JE. Guyton and Hall textbook of medical physiology. New York: Elsevier Health Sciences; 2010.
- Magee AC. Biological responses of young rats fed diets containing genistin and genistein. Journal of Nutrition. 1963; 80:151-6.
- Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H. Reproductive effects in male and female rats of neonatal exposure to genistein. Reproductive Toxicology. 2001; 15(4):399-411. doi: 10.1016/s0890-6238(01)00141-1
- Matsumoto AM, Paulsen CA, Bremner WJ. Stimulation of sperm production by human luteinizing hormone in gonadotropin-suppressed normal men. The Journal of Clinical Endocrinology & Metabolism. 1984; 59(5):882-7. doi: 10.1210/jcem-59-5-882
- Morovvati H, Najafzadehvarzi H, Rashidi K. Effect of urtica dioica extract on histological and histometrical changes of testis of hamster after testosteron administration. Zahedan Journal of Research in Medical Sciences. 2013; 15(11):4-8.
- Zhang GY, Gu YQ, Wang XH, Cui YG, Bremner WJ. A clinical trial of injectable testosterone undecanoate as a potential male contraceptive in normal Chinese men. The Journal of Clinical Endocrinology & Metabolism. 1999; 84(10):3642-7. doi: 10.1210/jc.84.10.3642
- Meachem SJ, Schlatt S, Ruwanpura SM, Stanton PG. The effect of testosterone, dihydrotestosterone and oestradiol on the re-initiation of spermatogenesis in the adult photoinhibited Djungarian hamster. Journal of Endocrinology. 2007; 192(3):553-61. doi: 10.1677/joe-06-0136
- Zirkin BR, Santulli R, Awoniyi CA, Ewing LL. Maintenance of advanced spermatogenic cells in the adult rat testis: Quantitative relationship to testosterone concentration within the testis. Endocrinology. 1989; 124(6):3043-9. doi: 10.1210/endo-124-6-3043
- Meachem SJ, Stanton PG, Schlatt S. Follicle-stimulating hormone regulates both Sertoli cell and spermatogonial populations in the adult photoinhibited Djungarian hamster testis. Biology of Reproduction. 2005; 72(5):1187-93. doi: 10.1095/biolreprod.104.039321
- Cayatte C, Pons C, Guigonis JM, Pizzol J, Elies L, Kennel P, et al. Protein profiling of rat ventral prostate following chronic finasteride administration identification and localization of a novel putative androgen-regulated protein. Molecular & Cellular Proteomics. 2006; 5(11):2031-43. doi: 10.1074/mcp.m600165-mcp200
- Lieberman SE. Are the differences between estradiol and other estrogens, naturally occurring or synthetic, merely semantical. The Journal of Clinical Endocrinology & Metabolism. 1996; 81(2):850-1. doi: 10.1210/jc.81.2.850
- Nonclercq D, Reverse D, Toubeau G, Beckers JF, Sulon J, Laurent G, et al. In situ demonstration of germinal cell apoptosis during diethylstilbestrol-induced testis regression in adult male Syrian hamsters. Biology of Reproduction. 1996; 55(6):1368-76. doi: 10.1095/biolreprod55.6.1368
Type of Study: Original |
Received: 2016/08/2 | Accepted: 2017/01/13 | Published: 2017/07/1
Received: 2016/08/2 | Accepted: 2017/01/13 | Published: 2017/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |